HDL promotes rapid atherosclerosis regression in mice and alters inflammatory properties of plaque monocyte-derived cells
- PMID: 21482781
- PMCID: PMC3084076
- DOI: 10.1073/pnas.1016086108
HDL promotes rapid atherosclerosis regression in mice and alters inflammatory properties of plaque monocyte-derived cells
Erratum in
- Proc Natl Acad Sci U S A. 2011 Aug 23;108(34):14371
Abstract
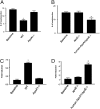
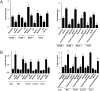
HDL cholesterol (HDL-C) plasma levels are inversely related to cardiovascular disease risk. Previous studies have shown in animals and humans that HDL promotes regression of atherosclerosis. We hypothesized that this was related to an ability to promote the loss of monocyte-derived cells (CD68(+), primarily macrophages and macrophage foam cells) from plaques. To test this hypothesis, we used an established model of atherosclerosis regression in which plaque-bearing aortic arches from apolipoprotein E-deficient (apoE(-/-)) mice (low HDL-C, high non-HDL-C) were transplanted into recipient mice with differing levels of HDL-C and non-HDL-C: C57BL6 mice (normal HDL-C, low non-HDL-C), apoAI(-/-) mice (low HDL-C, low non-HDL-C), or apoE(-/-) mice transgenic for human apoAI (hAI/apoE(-/-); normal HDL-C, high non-HDL-C). Remarkably, despite persistent elevated non-HDL-C in hAI/apoE(-/-) recipients, plaque CD68(+) cell content decreased by >50% by 1 wk after transplantation, whereas there was little change in apoAI(-/-) recipient mice despite hypolipidemia. The decreased content of plaque CD68(+) cells after HDL-C normalization was associated with their emigration and induction of their chemokine receptor CCR7. Furthermore, in CD68(+) cells laser-captured from the plaques, normalization of HDL-C led to decreased expression of inflammatory factors and enrichment of markers of the M2 (tissue repair) macrophage state. Again, none of these beneficial changes were observed in the apoAI(-/-) recipients, suggesting a major requirement for reverse cholesterol transport for the beneficial effects of HDL. Overall, these results establish HDL as a regulator in vivo of the migratory and inflammatory properties of monocyte-derived cells in mouse atherosclerotic plaques, and highlight the phenotypic plasticity of these cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


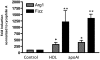

References
-
- Castelli WP, et al. Incidence of coronary heart disease and lipoprotein cholesterol levels. The Framingham Study. JAMA. 1986;256:2835–2838. - PubMed
-
- Feig JE, Shamir R, Fisher EA. Atheroprotective effects of HDL: Beyond reverse cholesterol transport. Curr Drug Targets. 2008;9:196–203. - PubMed
-
- Franceschini G. Epidemiologic evidence for high-density lipoprotein cholesterol as a risk factor for coronary artery disease. Am J Cardiol. 2001;88(12A):9N–13N. - PubMed
-
- Gordon DJ, Rifkind BM. High-density lipoprotein—The clinical implications of recent studies. N Engl J Med. 1989;321:1311–1316. - PubMed
-
- Miller NE, Miller GJ. Letter: High-density lipoprotein and atherosclerosis. Lancet. 1975;1:1033. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

