Mice lacking phosphatase PP2A subunit PR61/B'delta (Ppp2r5d) develop spatially restricted tauopathy by deregulation of CDK5 and GSK3beta
- PMID: 21482799
- PMCID: PMC3084077
- DOI: 10.1073/pnas.1018777108
Mice lacking phosphatase PP2A subunit PR61/B'delta (Ppp2r5d) develop spatially restricted tauopathy by deregulation of CDK5 and GSK3beta
Abstract
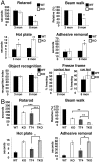
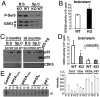
Functional diversity of protein phosphatase 2A (PP2A) enzymes mainly results from their association with distinct regulatory subunits. To analyze the functions of one such holoenzyme in vivo, we generated mice lacking PR61/B'δ (B56δ), a subunit highly expressed in neural tissues. In PR61/B'δ-null mice the microtubule-associated protein tau becomes progressively phosphorylated at pathological epitopes in restricted brain areas, with marked immunoreactivity for the misfolded MC1-conformation but without neurofibrillary tangle formation. Behavioral tests indicated impaired sensorimotor but normal cognitive functions. These phenotypical characteristics were further underscored in PR61/B'δ-null mice mildly overexpressing human tau. PR61/B'δ-containing PP2A (PP2A(T61δ)) poorly dephosphorylates tau in vitro, arguing against a direct dephosphorylation defect. Rather, the activity of glycogen synthase kinase-3β, a major tau kinase, was found increased, with decreased phosphorylation of Ser-9, a putative cyclin-dependent kinase 5 (CDK5) target. Accordingly, CDK5 activity is decreased, and its cellular activator p35, strikingly absent in the affected brain areas. As opposed to tau, p35 is an excellent PP2A(T61δ) substrate. Our data imply a nonredundant function for PR61/B'δ in phospho-tau homeostasis via an unexpected spatially restricted mechanism preventing p35 hyperphosphorylation and its subsequent degradation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Janssens V, Longin S, Goris J. PP2A holoenzyme assembly: In cauda venenum (the sting is in the tail) Trends Biochem Sci. 2008;33:113–121. - PubMed
-
- Eichhorn PJ, Creyghton MP, Bernards R. Protein phosphatase 2A regulatory subunits and cancer. Biochim Biophys Acta. 2009;1795:1–15. - PubMed
-
- Janssens V, Goris J, Van Hoof C. PP2A: The expected tumor suppressor. Curr Opin Genet Dev. 2005;15:34–41. - PubMed
-
- Tian Q, Wang J. Role of serine/threonine protein phosphatase in Alzheimer’s disease. Neurosignals. 2002;11:262–269. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

