Cisplatin binds human copper chaperone Atox1 and promotes unfolding in vitro
- PMID: 21482801
- PMCID: PMC3084073
- DOI: 10.1073/pnas.1012899108
Cisplatin binds human copper chaperone Atox1 and promotes unfolding in vitro
Abstract
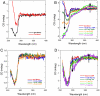
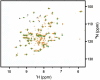
Cisplatin (cisPt), Pt(NH(3))(2)Cl(2), is a cancer drug believed to kill cells via DNA binding and damage. Recent work has implied that the cellular copper (Cu) transport machinery may be involved in cisPt cell export and drug resistance. Normally, the Cu chaperone Atox1 binds Cu(I) via two cysteines and delivers the metal to metal-binding domains of ATP7B; the ATP7B domains then transfer the metal to the Golgi lumen for loading on cuproenzymes. Here, we use spectroscopic methods to test if cisPt interacts with purified Atox1 in solution in vitro. We find that cisPt binds to Atox1's metal-binding site regardless of the presence of Cu or not: When Cu is bound to Atox1, the near-UV circular dichroism signals indicate Cu-Pt interactions. From NMR data, it is evident that cisPt binds to the folded protein. CisPt-bound Atox1 is however not stable over time and the protein begins to unfold and aggregate. The reaction rates are limited by slow cisPt dechlorination. CisPt-induced unfolding of Atox1 is specific because this effect was not observed for two unrelated proteins that also bind cisPt. Our study demonstrates that Atox1 is a candidate for cisPt drug resistance: By binding to Atox1 in the cytoplasm, cisPt transport to DNA may be blocked. In agreement with this model, cell line studies demonstrate a correlation between Atox1 expression levels, and cisplatin resistance.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Determinants for simultaneous binding of copper and platinum to human chaperone Atox1: hitchhiking not hijacking.PLoS One. 2013 Jul 30;8(7):e70473. doi: 10.1371/journal.pone.0070473. Print 2013. PLoS One. 2013. PMID: 23936210 Free PMC article.
-
Interaction between the anticancer drug Cisplatin and the copper chaperone Atox1 in human melanoma cells.Protein Pept Lett. 2014;21(1):63-8. doi: 10.2174/09298665113209990036. Protein Pept Lett. 2014. PMID: 23988033
-
Conserved residues modulate copper release in human copper chaperone Atox1.Proc Natl Acad Sci U S A. 2008 Aug 12;105(32):11158-63. doi: 10.1073/pnas.0802928105. Epub 2008 Aug 6. Proc Natl Acad Sci U S A. 2008. PMID: 18685091 Free PMC article.
-
An expanding range of functions for the copper chaperone/antioxidant protein Atox1.Antioxid Redox Signal. 2013 Sep 20;19(9):945-57. doi: 10.1089/ars.2012.5086. Epub 2013 Feb 6. Antioxid Redox Signal. 2013. PMID: 23249252 Free PMC article. Review.
-
ATOX1: a novel copper-responsive transcription factor in mammals?Int J Biochem Cell Biol. 2009 Jun;41(6):1233-6. doi: 10.1016/j.biocel.2008.08.001. Epub 2008 Aug 7. Int J Biochem Cell Biol. 2009. PMID: 18761103 Review.
Cited by
-
Copper transporter 2 regulates intracellular copper and sensitivity to cisplatin.Metallomics. 2014 Mar;6(3):654-61. doi: 10.1039/c3mt00331k. Epub 2014 Feb 13. Metallomics. 2014. PMID: 24522273 Free PMC article.
-
Interaction of classical platinum agents with the monomeric and dimeric Atox1 proteins: a molecular dynamics simulation study.Int J Mol Sci. 2013 Dec 20;15(1):75-99. doi: 10.3390/ijms15010075. Int J Mol Sci. 2013. PMID: 24362578 Free PMC article.
-
A re-evaluation of the role of hCTR1, the human high-affinity copper transporter, in platinum-drug entry into human cells.Mol Pharmacol. 2013 Jun;83(6):1237-46. doi: 10.1124/mol.113.085068. Epub 2013 Mar 29. Mol Pharmacol. 2013. PMID: 23543413 Free PMC article.
-
Roles of Atox1 and p53 in the trafficking of copper-64 to tumor cell nuclei: implications for cancer therapy.J Biol Inorg Chem. 2014 Mar;19(3):427-38. doi: 10.1007/s00775-013-1087-0. Epub 2014 Jan 21. J Biol Inorg Chem. 2014. PMID: 24445997 Free PMC article.
-
Roles of Copper-Binding Proteins in Breast Cancer.Int J Mol Sci. 2017 Apr 20;18(4):871. doi: 10.3390/ijms18040871. Int J Mol Sci. 2017. PMID: 28425924 Free PMC article. Review.
References
-
- Ivanov AI, et al. Cisplatin binding sites on human albumin. J Biol Chem. 1998;273:14721–14730. - PubMed
-
- Zhao T, King FL. Direct determination of the primary binding site of cisplatin on cytochrome C by mass spectrometry. J Am Soc Mass Spectrom. 2009;20:1141–1147. - PubMed
-
- Calderone V, Casini A, Mangani S, Messori L, Orioli PL. Structural investigation of cisplatin-protein interactions: Selective platination of His19 in a cuprozinc superoxide dismutase. Angew Chem Int Ed Engl. 2006;45:1267–1269. - PubMed
-
- Casini A, et al. ESI mass spectrometry and X-ray diffraction studies of adducts between anticancer platinum drugs and hen egg white lysozyme. Chem Commun (Camb) 2007;14:156–158. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

