Biosynthesis of hemiketal eicosanoids by cross-over of the 5-lipoxygenase and cyclooxygenase-2 pathways
- PMID: 21482803
- PMCID: PMC3084063
- DOI: 10.1073/pnas.1019473108
Biosynthesis of hemiketal eicosanoids by cross-over of the 5-lipoxygenase and cyclooxygenase-2 pathways
Abstract
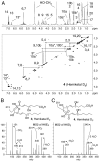
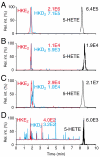
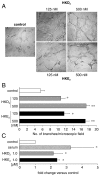
The prostaglandin and leukotriene families of lipid mediators are formed via two distinct biosynthetic pathways that are initiated by the oxygenation of arachidonic acid by either cyclooxygenase-2 (COX-2) or 5-lipoxygenase (5-LOX), respectively. The 5-LOX product 5S-hydroxyeicosatetraenoic acid, however, can also serve as an efficient substrate for COX-2, forming a bicyclic diendoperoxide with structural similarities to the arachidonic acid-derived prostaglandin endoperoxide PGH(2) [Schneider C, et al. (2006) J Am Chem Soc 128:720-721]. Here we identify two cyclic hemiketal (HK) eicosanoids, HKD(2) and HKE(2), as the major nonenzymatic rearrangement products of the diendoperoxide using liquid chromatography-mass spectrometry analyses as well as UV and NMR spectroscopy. HKD(2) and HKE(2) are furoketals formed by spontaneous cyclization of their respective 8,9-dioxo-5S,11R,12S,15S-tetrahydroxy- or 11,12-dioxo-5S,8S,9S,15S-tetrahydroxy-eicosadi-6E,13E-enoic acid precursors, resulting from opening of the 9S,11R- and 8S,12S-peroxide rings of the diendoperoxide. Furthermore, the diendoperoxide is an efficient substrate for the hematopoietic type of prostaglandin D synthase resulting in formation of HKD(2), equivalent to the enzymatic transformation of PGH(2) to PGD(2). HKD(2) and HKE(2) were formed in human blood leukocytes activated with bacterial lipopolysaccharide and calcium ionophore A23187, and biosynthesis was blocked by inhibitors of 5-LOX or COX-2. HKD(2) and HKE(2) stimulated migration and tubulogenesis of microvascular endothelial cells, implicating a proangiogenic role of the hemiketals in inflammatory sites that involve expression of 5-LOX and COX-2. Identification of the highly oxygenated hemiketal eicosanoids provides evidence for a previously unrecognized biosynthetic cross-over of the 5-LOX and COX-2 pathways.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Funk CD. Prostaglandins and leukotrienes: Advances in eicosanoid biology. Science. 2001;294:1871–1875. - PubMed
-
- Shimizu T. Lipid mediators in health and disease: Enzymes and receptors as therapeutic targets for the regulation of immunity and inflammation. Annu Rev Pharmacol Toxicol. 2009;49:123–150. - PubMed
-
- Murphy RC, Gijon MA. Biosynthesis and metabolism of leukotrienes. Biochem J. 2007;405:379–395. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

