Interstrand dipole-dipole interactions can stabilize the collagen triple helix
- PMID: 21482820
- PMCID: PMC3123058
- DOI: 10.1074/jbc.M110.199984
Interstrand dipole-dipole interactions can stabilize the collagen triple helix
Abstract
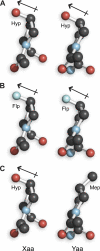
The amino acid sequence of collagen is composed of GlyXaaYaa repeats. A prevailing paradigm maintains that stable collagen triple helices form when (2S)-proline (Pro) or Pro derivatives that prefer the C(γ)-endo ring pucker are in the Xaa position and Pro derivatives that prefer the C(γ)-exo ring pucker are in the Yaa position. Anomalously, an amino acid sequence in an invertebrate collagen has (2S,4R)-4-hydroxyproline (Hyp), a C(γ)-exo-puckered Pro derivative, in the Xaa position. In certain contexts, triple helices with Hyp in the Xaa position are now known to be hyperstable. Most intriguingly, the sequence (GlyHypHyp)(n) forms a more stable triple helix than does the sequence (GlyProHyp)(n). Competing theories exist for the physicochemical basis of the hyperstability of (GlyHypHyp)(n) triple helices. By synthesizing and analyzing triple helices with different C(γ)-exo-puckered proline derivatives in the Xaa and Yaa positions, we conclude that interstrand dipole-dipole interactions are the primary determinant of their additional stability. These findings provide a new framework for understanding collagen stability.
Figures
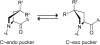


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

