A novel transcription factor, ERD15 (Early Responsive to Dehydration 15), connects endoplasmic reticulum stress with an osmotic stress-induced cell death signal
- PMID: 21482825
- PMCID: PMC3103375
- DOI: 10.1074/jbc.M111.233494
A novel transcription factor, ERD15 (Early Responsive to Dehydration 15), connects endoplasmic reticulum stress with an osmotic stress-induced cell death signal
Abstract
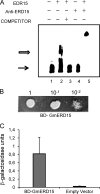
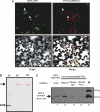
As in all other eukaryotic organisms, endoplasmic reticulum (ER) stress triggers the evolutionarily conserved unfolded protein response in soybean, but it also communicates with other adaptive signaling responses, such as osmotic stress-induced and ER stress-induced programmed cell death. These two signaling pathways converge at the level of gene transcription to activate an integrated cascade that is mediated by N-rich proteins (NRPs). Here, we describe a novel transcription factor, GmERD15 (Glycine max Early Responsive to Dehydration 15), which is induced by ER stress and osmotic stress to activate the expression of NRP genes. GmERD15 was isolated because of its capacity to stably associate with the NRP-B promoter in yeast. It specifically binds to a 187-bp fragment of the NRP-B promoter in vitro and activates the transcription of a reporter gene in yeast. Furthermore, GmERD15 was found in both the cytoplasm and the nucleus, and a ChIP assay revealed that it binds to the NRP-B promoter in vivo. Expression of GmERD15 in soybean protoplasts activated the NRP-B promoter and induced expression of the NRP-B gene. Collectively, these results support the interpretation that GmERD15 functions as an upstream component of stress-induced NRP-B-mediated signaling to connect stress in the ER to an osmotic stress-induced cell death signal.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

