HIV-1 p17 matrix protein interacts with heparan sulfate side chain of CD44v3, syndecan-2, and syndecan-4 proteoglycans expressed on human activated CD4+ T cells affecting tumor necrosis factor alpha and interleukin 2 production
- PMID: 21482826
- PMCID: PMC3103333
- DOI: 10.1074/jbc.M110.191270
HIV-1 p17 matrix protein interacts with heparan sulfate side chain of CD44v3, syndecan-2, and syndecan-4 proteoglycans expressed on human activated CD4+ T cells affecting tumor necrosis factor alpha and interleukin 2 production
Abstract
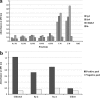
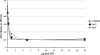
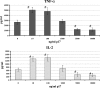
HIV-1 p17 contains C- and N-terminal sequences with positively charged residues and a consensus cluster for heparin binding. We have previously demonstrated by affinity chromatography that HIV-1 p17 binds strongly to heparin-agarose at physiological pH and to human activated CD4(+) T cells. In this study we demonstrated that the viral protein binds to heparan sulfate side chains of syndecan-2, syndecan-4, and CD44v3 purified from HeLa cells and that these heparan sulfate proteoglycans (HSPGs) co-localize with HIV-1 p17 on activated human CD4(+) T cells by confocal fluorescence analysis. Moreover, we observed a stimulatory or inhibitory activity when CD4(+) T cells were activated with mitogens together with nanomolar or micromolar concentrations of the matrix protein.
Figures

Similar articles
-
The HIV matrix protein p17 promotes the activation of human hepatic stellate cells through interactions with CXCR2 and Syndecan-2.PLoS One. 2014 Apr 15;9(4):e94798. doi: 10.1371/journal.pone.0094798. eCollection 2014. PLoS One. 2014. PMID: 24736615 Free PMC article.
-
HIV-1 p17 binds heparan sulfate proteoglycans to activated CD4(+) T cells.Virus Res. 2008 Mar;132(1-2):25-32. doi: 10.1016/j.virusres.2007.10.006. Epub 2007 Nov 26. Virus Res. 2008. PMID: 18036696
-
Heparin and heparan sulfate proteoglycans promote HIV-1 p17 matrix protein oligomerization: computational, biochemical and biological implications.Sci Rep. 2019 Oct 31;9(1):15768. doi: 10.1038/s41598-019-52201-w. Sci Rep. 2019. PMID: 31673058 Free PMC article.
-
HIV-1 Matrix Protein p17 and its Receptors.Curr Drug Targets. 2016;17(1):23-32. doi: 10.2174/1389450116666150825110840. Curr Drug Targets. 2016. PMID: 26302809 Review.
-
Heparan Sulfate Proteoglycans in Human Colorectal Cancer.Anal Cell Pathol (Amst). 2018 Jun 20;2018:8389595. doi: 10.1155/2018/8389595. eCollection 2018. Anal Cell Pathol (Amst). 2018. PMID: 30027065 Free PMC article. Review.
Cited by
-
TLR10 Senses HIV-1 Proteins and Significantly Enhances HIV-1 Infection.Front Immunol. 2019 Mar 15;10:482. doi: 10.3389/fimmu.2019.00482. eCollection 2019. Front Immunol. 2019. PMID: 30930906 Free PMC article.
-
Social, microbial, and immune factors linking bacterial vaginosis and infectious diseases.J Clin Invest. 2025 Jun 2;135(11):e184322. doi: 10.1172/JCI184322. eCollection 2025 Jun 2. J Clin Invest. 2025. PMID: 40454473 Free PMC article. Review.
-
Syndecan 4 Upregulation on Activated Langerhans Cells Counteracts Langerin Restriction to Facilitate Hepatitis C Virus Transmission.Front Immunol. 2020 Mar 27;11:503. doi: 10.3389/fimmu.2020.00503. eCollection 2020. Front Immunol. 2020. PMID: 32292405 Free PMC article.
-
The HIV matrix protein p17 induces hepatic lipid accumulation via modulation of nuclear receptor transcriptoma.Sci Rep. 2015 Oct 15;5:15403. doi: 10.1038/srep15403. Sci Rep. 2015. PMID: 26469385 Free PMC article.
-
The HIV matrix protein p17 promotes the activation of human hepatic stellate cells through interactions with CXCR2 and Syndecan-2.PLoS One. 2014 Apr 15;9(4):e94798. doi: 10.1371/journal.pone.0094798. eCollection 2014. PLoS One. 2014. PMID: 24736615 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

