Validation of a novel staging system for disease-specific survival in patients with breast cancer treated with neoadjuvant chemotherapy
- PMID: 21482989
- PMCID: PMC3107758
- DOI: 10.1200/JCO.2010.31.8469
Validation of a novel staging system for disease-specific survival in patients with breast cancer treated with neoadjuvant chemotherapy
Abstract
Purpose: We previously described a novel breast cancer staging system for assessing prognosis after neoadjuvant chemotherapy on the basis of pretreatment clinical stage (CS), estrogen receptor status (E), grade (G), and post-treatment pathologic stage (PS). This clinical-pathologic stage (CPS) + EG staging system assigned and summed points for each factor, allowing for better determination of breast cancer-specific survival than CS or PS alone. The current study was undertaken to validate this staging system using internal and external cohorts.
Methods: We identified an internal cohort of 804 patients treated with neoadjuvant chemotherapy at our institution from 2003 to 2005 and an external cohort of 165 patients treated at another institution. Clinicopathologic characteristics, treatment regimens, and patient outcomes were assessed. Outcomes were stratified by CPS + EG score.
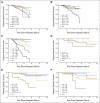
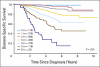
Results: Five-year disease-specific survival (DSS) for the internal cohort was 77% (95% CI, 72 to 82) at a median follow-up of 3.4 years (range, 0.3 to 5.9 years). Five-year DSS for the external cohort was 86% (95% CI, 79 to 91) at a median follow-up of 4.7 years (range, 0.5 to 10.5 years). The ability of the CPS + EG score to stratify outcomes was confirmed in both the internal and external cohorts. Application of the CPS + EG staging system facilitated more refined categorization of patients into prognostic subgroups by outcome than presenting CS or final PS as defined by the American Joint Committee on Cancer (AJCC) staging system.
Conclusion: The current study validates the CPS + EG staging system in two independent cohorts. We recommend that biologic markers and response to treatment be incorporated into revised versions of the AJCC staging system for patients receiving neoadjuvant chemotherapy.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures

References
-
- Kuerer HM, Newman LA, Smith TL, et al. Clinical course of breast cancer patients with complete pathologic primary tumor and axillary lymph node response to doxorubicin-based neoadjuvant chemotherapy. J Clin Oncol. 1999;17:460–469. - PubMed
-
- Fisher B, Bryant J, Wolmark N, et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol. 1998;16:2672–2685. - PubMed
-
- Bonadonna G, Valagussa P, Brambilla C, et al. Primary chemotherapy in operable breast cancer: Eight-year experience at the Milan Cancer Institute. J Clin Oncol. 1998;16:93–100. - PubMed
-
- Carey LA, Metzger R, Dees EC, et al. American Joint Committee on Cancer tumor-node-metastasis stage after neoadjuvant chemotherapy and breast cancer outcome. J Natl Cancer Inst. 2005;97:1137–1142. - PubMed
-
- Jeruss JS, Mittendorf EA, Tucker SL, et al. Combined use of clinical and pathologic staging variables to define outcomes for breast cancer patients treated with neoadjuvant therapy. J Clin Oncol. 2008;26:246–252. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

