Dissection of the influenza A virus endocytic routes reveals macropinocytosis as an alternative entry pathway
- PMID: 21483486
- PMCID: PMC3068995
- DOI: 10.1371/journal.ppat.1001329
Dissection of the influenza A virus endocytic routes reveals macropinocytosis as an alternative entry pathway
Abstract
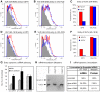
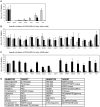
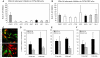
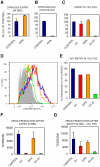
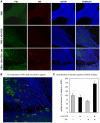
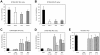
Influenza A virus (IAV) enters host cells upon binding of its hemagglutinin glycoprotein to sialylated host cell receptors. Whereas dynamin-dependent, clathrin-mediated endocytosis (CME) is generally considered as the IAV infection pathway, some observations suggest the occurrence of an as yet uncharacterized alternative entry route. By manipulating entry parameters we established experimental conditions that allow the separate analysis of dynamin-dependent and -independent entry of IAV. Whereas entry of IAV in phosphate-buffered saline could be completely inhibited by dynasore, a specific inhibitor of dynamin, a dynasore-insensitive entry pathway became functional in the presence of fetal calf serum. This finding was confirmed with the use of small interfering RNAs targeting dynamin-2. In the presence of serum, both IAV entry pathways were operational. Under these conditions entry could be fully blocked by combined treatment with dynasore and the amiloride derivative EIPA, the hallmark inhibitor of macropinocytosis, whereas either drug alone had no effect. The sensitivity of the dynamin-independent entry pathway to inhibitors or dominant-negative mutants affecting actomyosin dynamics as well as to a number of specific inhibitors of growth factor receptor tyrosine kinases and downstream effectors thereof all point to the involvement of macropinocytosis in IAV entry. Consistently, IAV particles and soluble FITC-dextran were shown to co-localize in cells in the same vesicles. Thus, in addition to the classical dynamin-dependent, clathrin-mediated endocytosis pathway, IAV enters host cells by a dynamin-independent route that has all the characteristics of macropinocytosis.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Patterson S, Oxford JS, Dourmashkin RR. Studies on the mechanism of influenza-virus entry into cells. J Gen Virol. 1979;43:223–229. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

