Results of antiretroviral treatment interruption and intensification in advanced multi-drug resistant HIV infection from the OPTIMA trial
- PMID: 21483491
- PMCID: PMC3069000
- DOI: 10.1371/journal.pone.0014764
Results of antiretroviral treatment interruption and intensification in advanced multi-drug resistant HIV infection from the OPTIMA trial
Abstract
Background: Guidance is needed on best medical management for advanced HIV disease with multidrug resistance (MDR) and limited retreatment options. We assessed two novel antiretroviral (ARV) treatment approaches in this setting.
Methods and findings: We conducted a 2×2 factorial randomized open label controlled trial in patients with a CD4 count≤300 cells/µl who had ARV treatment (ART) failure requiring retreatment, to two options (a) re-treatment with either standard (≤4 ARVs) or intensive (≥5 ARVs) ART and b) either treatment starting immediately or after a 12-week monitored ART interruption. Primary outcome was time to developing a first AIDS-defining event (ADE) or death from any cause. Analysis was by intention to treat. From 2001 to 2006, 368 patients were randomized. At baseline, mean age was 48 years, 2% were women, median CD4 count was 106/µl, mean viral load was 4.74 log(10) copies/ml, and 59% had a prior AIDS diagnosis. Median follow-up was 4.0 years in 1249 person-years of observation. There were no statistically significant differences in the primary composite outcome of ADE or death between re-treatment options of standard versus intensive ART (hazard ratio 1.17; CI 0.86-1.59), or between immediate retreatment initiation versus interruption before re-treatment (hazard ratio 0.93; CI 0.68-1.30), or in the rate of non-HIV associated serious adverse events between re-treatment options.
Conclusions: We did not observe clinical benefit or harm assessed by the primary outcome in this largest and longest trial exploring both ART interruption and intensification in advanced MDR HIV infection with poor retreatment options.
Trial registration: Clinicaltrials.gov NCT00050089.
Conflict of interest statement
Figures
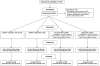

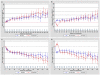
References
-
- d'Arminio Monforte A, Sabin CA, Phillips A, Sterne J, May M, et al. The changing incidence of AIDS events in patients receiving highly active antiretroviral therapy. Arch Intern Med. 2005;165:416–423. - PubMed
-
- Mocroft A, Ledergerber B, Katlama C, Kirk O, Reiss P, et al. Decline in the AIDS and death rates in the EuroSIDA study: an observational study. Lancet. 2003;362:22–29. - PubMed
-
- Antinori A, Zaccarelli M, Cingolani A, Forbici F, Rizzo MG, et al. Cross-resistance among nonnucleoside reverse transcriptase inhibitors limits recycling efavirenz after nevirapine failure. AIDS Res Hum Retroviruses. 2002;18:835–838. - PubMed
-
- Ledergerber B, Egger M, Opravil M, Telenti A, Hirschel B, et al. Clinical progression and virological failure on highly active antiretroviral therapy in HIV-1 patients: a prospective cohort study. Swiss HIV Cohort Study. Lancet. 1999;353:863–868. - PubMed
-
- Miller V, Larder BA. Mutational patterns in the HIV genome and cross-resistance following nucleoside and nucleotide analogue drug exposure. Antivir Ther. 2001;6(Suppl 3):25–44. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

