Low CD4/CD8 T-cell ratio associated with inflammatory arthropathy in human T-cell leukemia virus type I Tax transgenic mice
- PMID: 21483764
- PMCID: PMC3069963
- DOI: 10.1371/journal.pone.0018518
Low CD4/CD8 T-cell ratio associated with inflammatory arthropathy in human T-cell leukemia virus type I Tax transgenic mice
Abstract
Background: Human T-cell leukemia virus type I (HTLV-1) can cause an aggressive malignancy known as adult T-cell leukemia/lymphoma (ATL) as well as inflammatory diseases such as HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP). A transgenic mouse that expresses HTLV-1 Tax also develops T-cell leukemia/lymphoma and an inflammatory arthropathy that resembles rheumatoid arthritis. The aim of this study was to identify the primary T-cell subsets involved in the development of arthropathy in Tax transgenic mice.
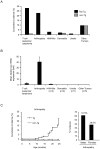
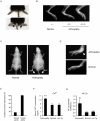
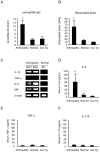
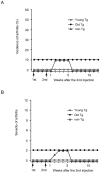
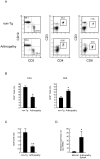
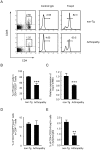
Principal findings: By 24 months of age, Tax transgenic mice developed severe arthropathy with a cumulative incidence of 22.8%. The pathological findings of arthropathy in Tax transgenic mice were similar to those seen in human rheumatoid arthritis or mouse models of rheumatoid arthritis, with synovial proliferation and a positive rheumatoid factor. Before the onset of spontaneous arthropathy, young and old Tax transgenic mice were not sensitive to collagen and did not develop arthritis after immunization with type II collagen. The arthropathic Tax transgenic mice showed a significantly decreased proportion of splenic CD4(+) T cells, whereas the proportion of splenic CD8(+) T cells was increased. Regulatory T cells (CD4(+)CD25(+)Foxp3(+)) were significantly decreased and CD8(+) T cells that expressed the chemokine receptor CCR4 (CD8(+)CCR4(+)) were significantly increased in arthropathic Tax transgenic mice. The expression of tax mRNA was strong in the spleen and joints of arthropathic mice, with a 40-fold increase compared with healthy transgenic mice.
Conclusions: Our findings reveal that Tax transgenic mice develop rheumatoid-like arthritis with proliferating synovial cells in the joints; however, the proportion of different splenic T-cell subsets in these mice was completely different from other commonly used animal models of rheumatoid arthritis. The crucial T-cell subsets in arthropathic Tax transgenic mice appear to resemble those in HAM/TSP patients rather than those in rheumatoid arthritis patients.
Conflict of interest statement
Figures


References
-
- Watanabe T. HTLV-1-associated diseases. Int J Hematol. 1997;66:257–278. - PubMed
-
- Verdonck K, Gonzalez E, Van Dooren S, Vandamme AM, Vanham G, et al. Human T-lymphotropic virus 1: recent knowledge about an ancient infection. Lancet Infect Dis. 2007;7:266–281. - PubMed
-
- Grassmann R, Aboud M, Jeang KT. Molecular mechanisms of cellular transformation by HTLV-1 Tax. Oncogene. 2005;24:5976–5985. - PubMed
-
- Matsuoka M, Jeang KT. Human T-cell leukaemia virus type 1 (HTLV-1) infectivity and cellular transformation. Nat Rev Cancer. 2007;7:270–280. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

