Mycobacterium tuberculosis induces an atypical cell death mode to escape from infected macrophages
- PMID: 21483832
- PMCID: PMC3069075
- DOI: 10.1371/journal.pone.0018367
Mycobacterium tuberculosis induces an atypical cell death mode to escape from infected macrophages
Abstract
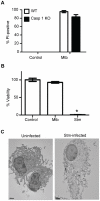
Background: Macrophage cell death following infection with Mycobacterium tuberculosis plays a central role in tuberculosis disease pathogenesis. Certain attenuated strains induce extrinsic apoptosis of infected macrophages but virulent strains of M. tuberculosis suppress this host response. We previously reported that virulent M. tuberculosis induces cell death when bacillary load exceeds ∼20 per macrophage but the precise nature of this demise has not been defined.
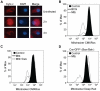
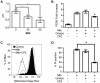
Methodology/principal findings: We analyzed the characteristics of cell death in primary murine macrophages challenged with virulent or attenuated M. tuberculosis complex strains. We report that high intracellular bacillary burden causes rapid and primarily necrotic death via lysosomal permeabilization, releasing hydrolases that promote Bax/Bak-independent mitochondrial damage and necrosis. Cell death was independent of cathepsins B or L and notable for ultrastructural evidence of damage to lipid bilayers throughout host cells with depletion of several host phospholipid species. These events require viable bacteria that can respond to intracellular cues via the PhoPR sensor kinase system but are independent of the ESX1 system.
Conclusions/significance: Cell death caused by virulent M. tuberculosis is distinct from classical apoptosis, pyroptosis or pyronecrosis. Mycobacterial genes essential for cytotoxicity are regulated by the PhoPR two-component system. This atypical death mode provides a mechanism for viable bacilli to exit host macrophages for spreading infection and the eventual transition to extracellular persistence that characterizes advanced pulmonary tuberculosis.
Conflict of interest statement
Figures


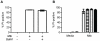

References
-
- Edwards KM, Cynamon MH, Voladri RK, Hager CC, DeStefano MS, et al. Iron-cofactored superoxide dismutase inhibits host responses to Mycobacterium tuberculosis. Am J Respir Crit Care Med. 2001;164:2213–2219. - PubMed
-
- Park JS, Tamayo MH, Gonzalez-Juarrero M, Orme IM, Ordway DJ. Virulent clinical isolates of Mycobacterium tuberculosis grow rapidly and induce cellular necrosis but minimal apoptosis in murine macrophages. J Leukoc Biol. 2006;79:80–86. - PubMed
-
- Lee J, Remold HG, Ieong MH, Kornfeld H. Macrophage apoptosis in response to high intracellular burden of Mycobacterium tuberculosis is mediated by a novel caspase-independent pathway. J Immunol. 2006;176:4267–4274. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

