The P2X1 receptor and platelet function
- PMID: 21484087
- PMCID: PMC3166991
- DOI: 10.1007/s11302-011-9224-0
The P2X1 receptor and platelet function
Abstract
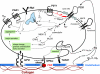
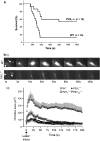
Extracellular nucleotides are ubiquitous signalling molecules, acting via the P2 class of surface receptors. Platelets express three P2 receptor subtypes, ADP-dependent P2Y1 and P2Y12 G-protein-coupled receptors and the ATP-gated P2X1 non-selective cation channel. Platelet P2X1 receptors can generate significant increases in intracellular Ca(2+), leading to shape change, movement of secretory granules and low levels of α(IIb)β(3) integrin activation. P2X1 can also synergise with several other receptors to amplify signalling and functional events in the platelet. In particular, activation of P2X1 receptors by ATP released from dense granules amplifies the aggregation responses to low levels of the major agonists, collagen and thrombin. In vivo studies using transgenic murine models show that P2X1 receptors amplify localised thrombosis following damage of small arteries and arterioles and also contribute to thromboembolism induced by intravenous co-injection of collagen and adrenaline. In vitro, under flow conditions, P2X1 receptors contribute more to aggregate formation on collagen-coated surfaces as the shear rate is increased, which may explain their greater contribution to localised thrombosis in arterioles compared to venules within in vivo models. Since shear increases substantially near sites of stenosis, anti-P2X1 therapy represents a potential means of reducing thrombotic events at atherosclerotic plaques.
Figures

Similar articles
-
Ca2+ influx through P2X1 receptors amplifies P2Y1 receptor-evoked Ca2+ signaling and ADP-evoked platelet aggregation.Mol Pharmacol. 2014 Sep;86(3):243-51. doi: 10.1124/mol.114.092528. Epub 2014 Jun 12. Mol Pharmacol. 2014. PMID: 24923466
-
The ATP-gated P2X1 ion channel acts as a positive regulator of platelet responses to collagen.Thromb Haemost. 2001 Nov;86(5):1264-71. Thromb Haemost. 2001. PMID: 11816716
-
The platelet P2 receptors as molecular targets for old and new antiplatelet drugs.Pharmacol Ther. 2005 Nov;108(2):180-92. doi: 10.1016/j.pharmthera.2005.03.009. Epub 2005 Jun 13. Pharmacol Ther. 2005. PMID: 15955565 Review.
-
A role of the fast ATP-gated P2X1 cation channel in thrombosis of small arteries in vivo.J Exp Med. 2003 Aug 18;198(4):661-7. doi: 10.1084/jem.20030144. Epub 2003 Aug 11. J Exp Med. 2003. PMID: 12913094 Free PMC article.
-
Nucleotide receptor signaling in platelets.J Thromb Haemost. 2006 Nov;4(11):2317-26. doi: 10.1111/j.1538-7836.2006.02192.x. J Thromb Haemost. 2006. PMID: 17059469 Review.
Cited by
-
Is glycoprotein VI involved in contractual negotiations?Res Pract Thromb Haemost. 2024 Jan 26;8(1):102329. doi: 10.1016/j.rpth.2024.102329. eCollection 2024 Jan. Res Pract Thromb Haemost. 2024. PMID: 38404946 Free PMC article. No abstract available.
-
Plant extracts inhibit ADP-induced platelet activation in humans: their potential therapeutic role as ADP antagonists.Purinergic Signal. 2014;10(2):233-9. doi: 10.1007/s11302-013-9393-0. Epub 2013 Nov 5. Purinergic Signal. 2014. PMID: 24190032 Free PMC article. Review.
-
The P2X1 receptor as a therapeutic target.Purinergic Signal. 2022 Dec;18(4):421-433. doi: 10.1007/s11302-022-09880-4. Epub 2022 Jul 11. Purinergic Signal. 2022. PMID: 35821454 Free PMC article. Review.
-
Lack of level I evidence on how to prevent infection after elective shoulder surgery.Knee Surg Sports Traumatol Arthrosc. 2018 Aug;26(8):2465-2480. doi: 10.1007/s00167-018-4832-7. Epub 2018 Jan 16. Knee Surg Sports Traumatol Arthrosc. 2018. PMID: 29340748
-
P2X7 receptors exhibit at least three modes of allosteric antagonism.Sci Adv. 2024 Oct 4;10(40):eado5084. doi: 10.1126/sciadv.ado5084. Epub 2024 Oct 4. Sci Adv. 2024. PMID: 39365862 Free PMC article.
References
-
- Palmer RK, Boyer JL, Schachter JB, Nicholas RA, Harden TK. Agonist action of adenosine triphosphates at the human P2Y1 receptor. Mol Pharmacol. 1998;54:1118–1123. - PubMed
-
- Takasaki J, Kamohara M, Saito T, Matsumoto M, Matsumoto S, Ohishi T, Soga T, Matsushime H, Furuichi K. Molecular cloning of the platelet P2TAC ADP receptor: pharmacological comparison with another ADP receptor, the P2Y1 receptor. Mol Pharmacol. 2001;60:432–439. - PubMed
-
- Waldo GL, Harden TK. Agonist binding and Gq-stimulating activities of the purified human P2Y1 receptor. Mol Pharmacol. 2004;65:426–436. - PubMed
-
- Hechler B, Vigne P, Leon C, Breittmayer JP, Gachet C, Frelin C. ATP derivatives are antagonists of the P2Y1 receptor: similarities to the platelet ADP receptor. Mol Pharmacol. 1998;53:727–733. - PubMed
-
- Leon C, Hechler B, Vial C, Leray C, Cazenave JP, Gachet C. The P2Y1 receptor is an ADP receptor antagonized by ATP and expressed in platelets and megakaryoblastic cells. FEBS Lett. 1997;403:26–30. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

