Pharmacochemistry of the platelet purinergic receptors
- PMID: 21484092
- PMCID: PMC3166987
- DOI: 10.1007/s11302-011-9216-0
Pharmacochemistry of the platelet purinergic receptors
Abstract
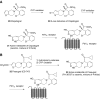
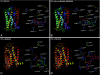
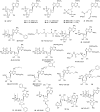
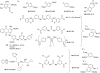
Platelets contain at least five purinergic G protein-coupled receptors, e.g., the pro-aggregatory P2Y(1) and P2Y(12) receptors, a P2Y(14) receptor (GPR105) of unknown function, and anti-aggregatory A(2A) and A(2B) adenosine receptor (ARs), in addition to the ligand-gated P2X1 ion channel. Probing the structure-activity relationships (SARs) of the P2X and P2Y receptors for extracellular nucleotides has resulted in numerous new agonist and antagonist ligands. Selective agents derived from known ligands and novel chemotypes can be used to help define the subtypes pharmacologically. Some of these agents have entered into clinical trials in spite of the challenges of drug development for these classes of receptors. The functional architecture of P2 receptors was extensively explored using mutagenesis and molecular modeling, which are useful tools in drug discovery. In general, novel drug delivery methods, prodrug approaches, allosteric modulation, and biased agonism would be desirable to overcome side effects that tend to occur even with receptor subtype-selective ligands. Detailed SAR analyses have been constructed for nucleotide and non-nucleotide ligands at the P2Y(1), P2Y(12), and P2Y(14) receptors. The thienopyridine antithrombotic drugs Clopidogrel and Prasugrel require enzymatic pre-activation in vivo and react irreversibly with the P2Y(12) receptor. There is much pharmaceutical development activity aimed at identifying reversible P2Y(12) receptor antagonists. The screening of chemically diverse compound libraries has identified novel chemotypes that act as competitive, non-nucleotide antagonists of the P2Y(1) receptor or the P2Y(12) receptor, and antithrombotic properties of the structurally optimized analogues were demonstrated. In silico screening at the A(2A) AR has identified antagonist molecules having novel chemotypes. Fluorescent and other reporter groups incorporated into ligands can enable new technology for receptor assays and imaging. The A(2A) agonist CGS21680 and the P2Y(1) receptor antagonist MRS2500 were derivatized for covalent attachment to polyamidoamine dendrimeric carriers of MW 20,000, and the resulting multivalent conjugates inhibited ADP-promoted platelet aggregation. In conclusion, a wide range of new pharmacological tools is available to control platelet function by interacting with cell surface purine receptors.
Figures


References
-
- Abbracchio M, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Fumagalli M, Gachet C, Jacobson KA, Weisman GA. International Union of Pharmacology LVIII. Update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev. 2006;58:281–341. - PMC - PubMed
-
- Marquez-Klaka B, Rettinger J, Nicke A. Inter-subunit disulfide cross-linking in homomeric and heteromeric P2X receptors. Eur Biophys J. 2009;3:329–338. - PubMed
-
- Armaz A, Sadtler S, Niculescu C, Rettinger J, Schmalzing G. Trimeric architecture of homomeric P2X2 and heteromeric P2X1+2 receptor subtypes. J Mol Biol. 2004;342:333–343. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

