Control of the heparosan N-deacetylation leads to an improved bioengineered heparin
- PMID: 21484210
- PMCID: PMC3124281
- DOI: 10.1007/s00253-011-3231-5
Control of the heparosan N-deacetylation leads to an improved bioengineered heparin
Abstract
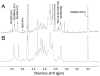
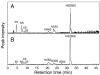
The production of the anticoagulant drug heparin from non-animal sources has a number of advantages over the current commercial production of heparin. These advantages include better source material availability, improved quality control, and reduced concerns about animal virus or prion impurities. A bioengineered heparin would have to be chemically and biologically equivalent to be substituted for animal-sourced heparin as a pharmaceutical. In an effort to produce bioengineered heparin that more closely resembles pharmaceutical heparin, we have investigated a key step in the process involving the N-deacetylation of heparosan. The extent of N-deacetylation directly affects the N-acetyl/N-sulfo ratio in bioengineered heparin and also impacts its molecular weight. Previous studies have demonstrated that the presence and quantity of N-acetylglucosamine in the nascent glycosaminoglycan chain, serving as the substrate for the subsequent enzymatic modifications (C5 epimerization and O-sulfonation), can impact the action of these enzymes and, thus, the content and distribution of iduronic acid and O-sulfo groups. In this study, we control the N-deacetylation of heparosan to produce a bioengineered heparin with an N-acetyl/N-sulfo ratio and molecular weight that is similar to animal-sourced pharmaceutical heparin. The structural composition and anticoagulant activity of the resultant bioengineered heparin was extensively characterized and compared to pharmaceutical heparin obtained from porcine intestinal mucosa.
Figures




Similar articles
-
Combinatorial one-pot chemoenzymatic synthesis of heparin.Carbohydr Polym. 2015 May 20;122:399-407. doi: 10.1016/j.carbpol.2014.10.054. Epub 2014 Nov 7. Carbohydr Polym. 2015. PMID: 25817684 Free PMC article.
-
Metabolic engineering of non-pathogenic Escherichia coli strains for the controlled production of low molecular weight heparosan and size-specific heparosan oligosaccharides.Biochim Biophys Acta Gen Subj. 2021 Jan;1865(1):129765. doi: 10.1016/j.bbagen.2020.129765. Epub 2020 Oct 16. Biochim Biophys Acta Gen Subj. 2021. PMID: 33069832
-
Response surface optimization of the heparosan N-deacetylation in producing bioengineered heparin.J Biotechnol. 2011 Dec 10;156(3):188-96. doi: 10.1016/j.jbiotec.2011.08.013. Epub 2011 Sep 10. J Biotechnol. 2011. PMID: 21925548 Free PMC article.
-
Production methods for heparosan, a precursor of heparin and heparan sulfate.Carbohydr Polym. 2013 Mar 1;93(1):38-47. doi: 10.1016/j.carbpol.2012.04.046. Epub 2012 May 14. Carbohydr Polym. 2013. PMID: 23465899 Review.
-
Progress on the synthesis of bioengineered heparin as an alternative to animal-derived heparin active pharmaceutical ingredient.Carbohydr Polym. 2025 Nov 15;368(Pt 1):124099. doi: 10.1016/j.carbpol.2025.124099. Epub 2025 Jul 24. Carbohydr Polym. 2025. PMID: 40912801 Review.
Cited by
-
Preparation and application of a 'clickable' acceptor for enzymatic synthesis of heparin oligosaccharides.Carbohydr Res. 2013 May 3;372:30-4. doi: 10.1016/j.carres.2013.02.010. Epub 2013 Mar 1. Carbohydr Res. 2013. PMID: 23524108 Free PMC article.
-
Bioengineered heparins and heparan sulfates.Adv Drug Deliv Rev. 2016 Feb 1;97:237-49. doi: 10.1016/j.addr.2015.11.002. Epub 2015 Nov 10. Adv Drug Deliv Rev. 2016. PMID: 26555370 Free PMC article. Review.
-
Combinatorial one-pot chemoenzymatic synthesis of heparin.Carbohydr Polym. 2015 May 20;122:399-407. doi: 10.1016/j.carbpol.2014.10.054. Epub 2014 Nov 7. Carbohydr Polym. 2015. PMID: 25817684 Free PMC article.
-
Molecular Mass Characterization of Glycosaminoglycans with Different Degrees of Sulfation in Bioengineered Heparin Process by Size Exclusion Chromatography.Curr Anal Chem. 2012 Oct 1;8(4):506-511. doi: 10.2174/157341112803216753. Curr Anal Chem. 2012. PMID: 23258975 Free PMC article.
-
Microscale separation of heparosan, heparan sulfate, and heparin.Anal Biochem. 2013 Mar 15;434(2):215-7. doi: 10.1016/j.ab.2012.12.009. Epub 2012 Dec 21. Anal Biochem. 2013. PMID: 23262074 Free PMC article.
References
-
- Agnelli G, Piovella F, Buoncristiani P, Severi P, Pini M, D’Angelo A, Beltrametti C, Damiani M, Andrioli GC, Pugliese R, Iorio A, Brambilla G. Enoxaparin plus compression stockings compared with compression stockings alone in the prevention of venous thromboembolism after elective neurosurgery. N Engl J Med. 1998;339:80–85. - PubMed
-
- Casu B, Lindahl U. Structure and biological interactions of heparin and heparan sulfate. Adv Carbohydr Chem Biochem. 2001;57:159–206. - PubMed
-
- Casu B, Grazioli G, Razi N, Guerrini M, Naggi A, Torri G, Oreste P, Tursi F, Zoppetti G, Lindahl U. Heparin-like compounds prepared by chemical modification of capsular polysaccharide from E. coli K5. Carbohydr Res. 1994;263:271–284. - PubMed
-
- Cesaretti M, Luppi E, Maccari F, Volpi N. Isolation and characterization of a heparin with high anticoagulant activity from the clam Tapes philippinarum: evidence for the presence of a high content of antithrombin III binding site. Glycobiology. 2004;14:1275–1284. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

