ST2 deletion enhances innate and acquired immunity to murine mammary carcinoma
- PMID: 21484786
- PMCID: PMC3746127
- DOI: 10.1002/eji.201141417
ST2 deletion enhances innate and acquired immunity to murine mammary carcinoma
Abstract
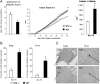
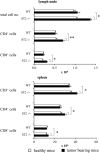
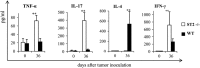
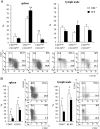
ST2 is a member of the IL-1 receptor family and IL-33 was recently identified as its natural ligand. The IL-33/ST2 pathway regulates Th1/Th2 immune responses in autoimmune and inflammatory conditions, but the role of ST2 signaling in tumor growth and metastasis has not been investigated. We aimed to investigate whether ST2 gene deletion affects tumor appearance, growth, and metastasis, and antitumor immunity in an experimental metastatic breast cancer model. Deletion of ST2 in BALB/c mice bearing mammary carcinoma attenuated tumor growth and metastasis, which was accompanied by increased serum levels of IL-17, IFN-γ, and TNF-α and decreased IL-4. Tumor-bearing ST2-/- mice had significantly higher percentages of activated CD27high CD11bhigh NK cells, CD69+ and KLRG- NK cells and higher cytotoxic activity of splenocytes, NK cells, and CD8+ T cells in vitro. A significantly higher number of NK cells expressing IFN-γ were found in ST2-/- mice compared with WT recipients. In vivo depletion of CD8+ or NK cells revealed a key role for NK cells in enhanced antitumor immunity in ST2-/- mice. We report for the first time that suppressed breast cancer progression and metastasis in mice lacking ST2 corresponds mainly with enhanced cytotoxic activity of NK cells, and increased systemic Th1/Th17 cytokines.
Copyright © 2011 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures


References
-
- Tominaga S. A putative protein of a growth specific cDNA from BALB/c-3T3 cells is highly similar to the extracellular portion of mouse interleukin 1 receptor. FEBS Lett. 1989;258:301–304. - PubMed
-
- Liew FY, Pitman NI, McInnes IB. Disease-associated function of IL-33: the new kid in the IL-1 family. Nat. Rev. Immunol. 2010;10:103–110. - PubMed
-
- Tago K, Noda T, Hayakawa M, Iwahana H, Yanagisawa K, Yashiro T, Tominaga S. Tissue distribution and subcellular localization of a variant form of the human ST2 gene product, ST2V. Biochem. Biophys. Res. Commun. 2001;285:1377–1383. - PubMed
-
- Trajkovic V, Sweet MJ, Xu D. T1/ST2-an IL-1 receptor-like modulator of immune responses. Cytokine Growth Factor Rev. 2004;15:87–95. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

