Sexually dimorphic expression of hypothalamic estrogen receptors α and β and Kiss1 in neonatal male and female rats
- PMID: 21484804
- PMCID: PMC3874381
- DOI: 10.1002/cne.22648
Sexually dimorphic expression of hypothalamic estrogen receptors α and β and Kiss1 in neonatal male and female rats
Abstract
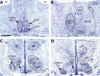
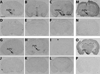
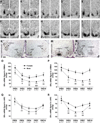
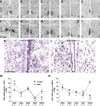
Release of gonadotropins in adult rodents is sex specific and dependent upon kisspeptin (Kiss1) neurons. This crucial pathway within the hypothalamic-pituitary-gonadal (HPG) axis is profoundly influenced by neonatal estrogens, which induce a male-like phenotype. Classically, estrogen activity is mediated via the estrogen receptors α and β (ERα and ERβ), but the relative roles each plays in organizing the sex-specific ontogeny of kisspeptin signaling pathways remain unresolved. Thus, the present study used in situ hybridization histochemistry (ISHH) to map the temporal and sexually dimorphic neonatal mRNA expression profiles of ERα, ERβ, and Kiss1 in the anterioventral periventricular nucleus (AVPV), medial preoptic area (MPOA), ventromedial nucleus (VMN), and arcuate nucleus (ARC), all regions critical for kisspeptin regulation of gonadotropin secretion. In general, females had higher levels of ERα, in all regions examined, a sex difference that persisted until postnatal day (PND) 19 except in the ARC. Males had significantly more ERβ expression in the AVPV at birth, but this sex difference was lost and then re-emerged on PND 19, with females having more than males. VMN ERβ levels were higher in females until PND 19. Kiss1 was not detectable until PND 11 in the anterior hypothalamus, but expression levels were equivalent at birth in the ARC. By PND 2, ARC ERα and Kiss1 levels were abundant, sexually dimorphic (higher in females), and, respectively, showed a U- and a bell-shaped pattern with age. Sex differences in ARC Kiss1 expression provide evidence that Kiss1 may play a role in the sexual dimorphic organization of the neonatal brain. These detailed profiles of neonatal Kiss1 and ERs mRNA levels will help elucidate the relative roles each plays in the sex-specific, estrogen-dependent organization of gonadotropin signaling pathways.
Copyright © 2011 Wiley-Liss, Inc.
Figures




Similar articles
-
Neonatal Bisphenol A exposure alters sexually dimorphic gene expression in the postnatal rat hypothalamus.Neurotoxicology. 2012 Jan;33(1):23-36. doi: 10.1016/j.neuro.2011.11.002. Epub 2011 Nov 9. Neurotoxicology. 2012. PMID: 22101008 Free PMC article.
-
Sex-specific expression of estrogen receptors α and β and Kiss1 in the postnatal rat amygdala.J Comp Neurol. 2013 Feb 1;521(2):465-78. doi: 10.1002/cne.23185. J Comp Neurol. 2013. PMID: 22791648 Free PMC article.
-
Sex Differences in Steroid Receptor Coexpression and Circadian-Timed Activation of Kisspeptin and RFRP-3 Neurons May Contribute to the Sexually Dimorphic Basis of the LH Surge.Endocrinology. 2017 Oct 1;158(10):3565-3578. doi: 10.1210/en.2017-00405. Endocrinology. 2017. PMID: 28938464 Free PMC article.
-
Gonadal and nongonadal regulation of sex differences in hypothalamic Kiss1 neurones.J Neuroendocrinol. 2010 Jul;22(7):682-91. doi: 10.1111/j.1365-2826.2010.02030.x. Epub 2010 May 12. J Neuroendocrinol. 2010. PMID: 20492362 Free PMC article. Review.
-
Kisspeptin signalling in the brain: steroid regulation in the rodent and ewe.Brain Res Rev. 2008 Mar;57(2):288-98. doi: 10.1016/j.brainresrev.2007.04.002. Epub 2007 Apr 19. Brain Res Rev. 2008. PMID: 17509691 Review.
Cited by
-
The decline in pulsatile GnRH release, as reflected by circulating LH concentrations, during the infant-juvenile transition in the agonadal male rhesus monkey (Macaca mulatta) is associated with a reduction in kisspeptin content of KNDy neurons of the arcuate nucleus in the hypothalamus.Endocrinology. 2013 May;154(5):1845-53. doi: 10.1210/en.2012-2154. Epub 2013 Mar 22. Endocrinology. 2013. PMID: 23525220 Free PMC article.
-
Essential and sex-specific effects of mGluR5 in ventromedial hypothalamus regulating estrogen signaling and glucose balance.Proc Natl Acad Sci U S A. 2020 Aug 11;117(32):19566-19577. doi: 10.1073/pnas.2011228117. Epub 2020 Jul 27. Proc Natl Acad Sci U S A. 2020. PMID: 32719118 Free PMC article.
-
Epigenetic impacts of endocrine disruptors in the brain.Front Neuroendocrinol. 2017 Jan;44:1-26. doi: 10.1016/j.yfrne.2016.09.002. Epub 2016 Sep 20. Front Neuroendocrinol. 2017. PMID: 27663243 Free PMC article. Review.
-
Sex- and age-dependent effects of maternal organophosphate flame-retardant exposure on neonatal hypothalamic and hepatic gene expression.Reprod Toxicol. 2020 Jun;94:65-74. doi: 10.1016/j.reprotox.2020.04.001. Epub 2020 Apr 29. Reprod Toxicol. 2020. PMID: 32360330 Free PMC article.
-
Impact of Low Dose Oral Exposure to Bisphenol A (BPA) on the Neonatal Rat Hypothalamic and Hippocampal Transcriptome: A CLARITY-BPA Consortium Study.Endocrinology. 2016 Oct;157(10):3856-3872. doi: 10.1210/en.2016-1339. Epub 2016 Aug 29. Endocrinology. 2016. PMID: 27571134 Free PMC article.
References
-
- Amateau SK, Alt JJ, Stamps CL, McCarthy MM. Brain estradiol content in newborn rats: sex differences, regional heterogeneity, and possible de novo synthesis by the female telencephalon. Endocrinology. 2004;145:2906–2917. - PubMed
-
- Arai Y, Gorski RA. Critical exposure time for androgeni-zation of the developing hypothalamus in the female rat. Endocrinology. 1968;82:1010–1014. - PubMed
-
- Arnold AP, Gorski RA. Gonadal steroid induction of structural sex differences in the central nervous system. Ann Rev Neurosci. 1984;7:413–442. - PubMed
-
- Brown NM, Setchell KD. Animal models impacted by phytoestrogens in commercial chow: implications for pathways influenced by hormones. Lab Invest. 2001;81:735–747. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

