α4β2 nicotinic acetylcholine receptors in the early postnatal mouse superior cervical ganglion
- PMID: 21485013
- PMCID: PMC3141087
- DOI: 10.1002/dneu.20870
α4β2 nicotinic acetylcholine receptors in the early postnatal mouse superior cervical ganglion
Abstract
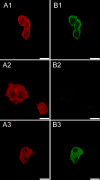
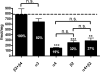
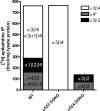
Heteropentameric nicotinic acetylcholine receptors (nAChR) mediate fast synaptic transmission in ganglia of the autonomic nervous system. It is undisputed that α3 and β4 are the predominant subunits in the superior cervical ganglion (SCG); however, reports on the presence of receptors that contain α4 have been controversial. Here, we have searched for the presence of α4-containing nAChRs in the postnatal rat and mouse SCG. We now show by immunoprecipitation combined with radioligand binding that α4-containing receptors constitute about 20% of hetero-oligomeric nAChRs in postnatal Day 3 (P3) mice. However, already by P9, the level of α4 approaches zero. In contrast, the number of α4-containing receptors is close to zero in the rat SCG at all times investigated. Deletion of the β2 subunit by using α5β2-double knockout (KO) mice removes all α4-containing receptors, suggesting that in the postnatal mouse SCG, α4 co-assembles only with β2 but not with β4. α4β2 receptors are, on the other hand, up-regulated in the SCG of P3 α5β4-double KO mice, where they make up about 50% of receptors that bind [(3) H]-epibatidine. Nonetheless, receptors on the surface of SCG neurons from α5β4-double KO mice maintained for one to two days in culture comprise <10% of α4β2 and >90% of α3β2, as determined by patch clamp recordings with α4β2- and α3β2-specific ligands. We propose that in the P3 SCG of wild type mice, α3β4 (±α5) represent about 62% of receptors, whereas 17% are α3β2β4, and 21% are α4β2 (±α5) receptors.
Copyright © 2011 Wiley Periodicals, Inc.
Figures



Similar articles
-
The role of the nAChR subunits α5, β2, and β4 on synaptic transmission in the mouse superior cervical ganglion.Physiol Rep. 2019 Mar;7(6):e14023. doi: 10.14814/phy2.14023. Physiol Rep. 2019. PMID: 30891952 Free PMC article.
-
Biochemical and functional properties of distinct nicotinic acetylcholine receptors in the superior cervical ganglion of mice with targeted deletions of nAChR subunit genes.Eur J Neurosci. 2010 Mar;31(6):978-93. doi: 10.1111/j.1460-9568.2010.07133.x. Epub 2010 Mar 3. Eur J Neurosci. 2010. PMID: 20377613 Free PMC article.
-
Heterogeneity of nicotinic cholinergic receptors in rat superior cervical and nodose Ganglia.Mol Pharmacol. 2006 Nov;70(5):1693-9. doi: 10.1124/mol.106.027458. Epub 2006 Aug 1. Mol Pharmacol. 2006. PMID: 16882879
-
Lack of dystrophin functionally affects α3β2/β4-nicotinic acethylcholine receptors in sympathetic neurons of dystrophic mdx mice.Neurobiol Dis. 2011 Feb;41(2):528-37. doi: 10.1016/j.nbd.2010.10.024. Epub 2010 Nov 5. Neurobiol Dis. 2011. PMID: 21056666
-
Nicotinic acetylcholine receptor-subunit mRNAs in the mouse superior cervical ganglion are regulated by development but not by deletion of distinct subunit genes.J Neurosci Res. 2008 Apr;86(5):972-81. doi: 10.1002/jnr.21559. J Neurosci Res. 2008. PMID: 17975828
Cited by
-
Alzheimer's Disease as a Membrane Disorder: Spatial Cross-Talk Among Beta-Amyloid Peptides, Nicotinic Acetylcholine Receptors and Lipid Rafts.Front Cell Neurosci. 2019 Jul 18;13:309. doi: 10.3389/fncel.2019.00309. eCollection 2019. Front Cell Neurosci. 2019. PMID: 31379503 Free PMC article. Review.
-
nAChRs gene expression and neuroinflammation in APPswe/PS1dE9 transgenic mouse.Sci Rep. 2021 May 6;11(1):9711. doi: 10.1038/s41598-021-89139-x. Sci Rep. 2021. Retraction in: Sci Rep. 2024 Nov 26;14(1):29316. doi: 10.1038/s41598-024-80730-6. PMID: 33958667 Free PMC article. Retracted.
-
Nicotinic regulation of energy homeostasis.Nicotine Tob Res. 2012 Nov;14(11):1270-90. doi: 10.1093/ntr/nts159. Epub 2012 Sep 18. Nicotine Tob Res. 2012. PMID: 22990212 Free PMC article. Review.
References
-
- Bansal A, Singer JH, Hwang BJ, Xu W, Beaudet A, Feller MB. Mice lacking specific nicotinic acetylcholine receptor subunits exhibit dramatically altered spontaneous activity patterns and reveal a limited role for retinal waves in forming ON and OFF circuits in the inner retina. J Neurosci. 2000;20:7672–7681. - PMC - PubMed
-
- Cartier GE, Yoshikami D, Gray WR, Luo S, Olivera BM, McIntosh JM. A new α-conotoxin which targets α3β2 nicotinic acetylcholine receptors. J Biol Chem. 1996;271:7522–7528. - PubMed
-
- Champtiaux N, Changeux J-P. Knockout and knockin mice to investigate the role of nicotinic receptors in the central nervous system. Progr Brain Res. 2004;145:235–251. - PubMed
-
- Champtiaux N, Gotti C, Cordero-Erausquin M, David DJ, Przybylski C, Lena C, Clementi F, Moretti M, Rossi FM, Le Novere N, McIntosh JM, Gardier AM, Changeux J-P. Subunit composition of functional nicotinic receptors in dopaminergic neurons investigated with knock-out mice. J Neurosci. 2003;23:7820–7829. - PMC - PubMed
-
- Chavez-Noriega LE, Crona JH, Washburn MS, Urrutia A, Elliott KJ, Johnson EC. Pharmacological characterization of recombinant human neuronal nicotinic acetylcholine receptors hα2β2, hα2β4, hα3β2, hα3β4, hα4β2, hα4β4, and hα7 expressed in Xenopus oocytes. J Pharmacol Exp Ther. 1997;280:346–356. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

