Selective adenosine A2A receptor agonists and antagonists protect against spinal cord injury through peripheral and central effects
- PMID: 21486435
- PMCID: PMC3096915
- DOI: 10.1186/1742-2094-8-31
Selective adenosine A2A receptor agonists and antagonists protect against spinal cord injury through peripheral and central effects
Abstract
Background: Permanent functional deficits following spinal cord injury (SCI) arise both from mechanical injury and from secondary tissue reactions involving inflammation. Enhanced release of adenosine and glutamate soon after SCI represents a component in the sequelae that may be responsible for resulting functional deficits. The role of adenosine A2A receptor in central ischemia/trauma is still to be elucidated. In our previous studies we have demonstrated that the adenosine A2A receptor-selective agonist CGS21680, systemically administered after SCI, protects from tissue damage, locomotor dysfunction and different inflammatory readouts. In this work we studied the effect of the adenosine A2A receptor antagonist SCH58261, systemically administered after SCI, on the same parameters. We investigated the hypothesis that the main action mechanism of agonists and antagonists is at peripheral or central sites.
Methods: Spinal trauma was induced by extradural compression of SC exposed via a four-level T5-T8 laminectomy in mouse. Three drug-dosing protocols were utilized: a short-term systemic administration by intraperitoneal injection, a chronic administration via osmotic minipump, and direct injection into the spinal cord.
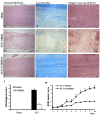
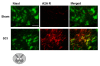
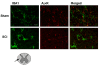
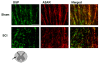
Results: SCH58261, systemically administered (0.01 mg/kg intraperitoneal. 1, 6 and 10 hours after SCI), reduced demyelination and levels of TNF-α, Fas-L, PAR, Bax expression and activation of JNK mitogen-activated protein kinase (MAPK) 24 hours after SCI. Chronic SCH58261 administration, by mini-osmotic pump delivery for 10 days, improved the neurological deficit up to 10 days after SCI. Adenosine A2A receptors are physiologically expressed in the spinal cord by astrocytes, microglia and oligodendrocytes. Soon after SCI (24 hours), these receptors showed enhanced expression in neurons. Both the A2A agonist and antagonist, administered intraperitoneally, reduced expression of the A2A receptor, ruling out the possibility that the neuroprotective effects of the A2A agonist are due to A2A receptor desensitization. When the A2A antagonist and agonist were centrally injected into injured SC, only SCH58261 appeared neuroprotective, while CGS21680 was ineffective.
Conclusions: Our results indicate that the A2A antagonist protects against SCI by acting on centrally located A2A receptors. It is likely that blockade of A2A receptors reduces excitotoxicity. In contrast, neuroprotection afforded by the A2A agonist may be primarily due to peripheral effects.
Figures





References
-
- Hagg T, Oudega M. Degenerative and spontaneous regenerative processes after spinal cord injury. J Neurotrauma. 2006;23:264–280. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

