Inflammatory mediators in breast cancer: coordinated expression of TNFα & IL-1β with CCL2 & CCL5 and effects on epithelial-to-mesenchymal transition
- PMID: 21486440
- PMCID: PMC3095565
- DOI: 10.1186/1471-2407-11-130
Inflammatory mediators in breast cancer: coordinated expression of TNFα & IL-1β with CCL2 & CCL5 and effects on epithelial-to-mesenchymal transition
Abstract
Background: The inflammatory chemokines CCL2 (MCP-1) & CCL5 (RANTES) and the inflammatory cytokines TNFα & IL-1β were shown to contribute to breast cancer development and metastasis. In this study, we wished to determine whether there are associations between these factors along stages of breast cancer progression, and to identify the possible implications of these factors to disease course.
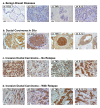
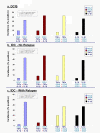
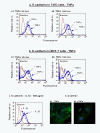
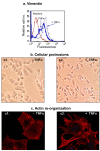
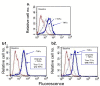
Methods: The expression of CCL2, CCL5, TNFα and IL-1β was determined by immunohistochemistry in patients diagnosed with: (1) Benign breast disorders (=healthy individuals); (2) Ductal Carcinoma In Situ (DCIS); (3) Invasive Ducal Carcinoma without relapse (IDC-no-relapse); (4) IDC-with-relapse. Based on the results obtained, breast tumor cells were stimulated by the inflammatory cytokines, and epithelial-to-mesenchymal transition (EMT) was determined by flow cytometry, confocal analyses and adhesion, migration and invasion experiments.
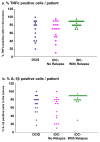
Results: CCL2, CCL5, TNFα and IL-1β were expressed at very low incidence in normal breast epithelial cells, but their incidence was significantly elevated in tumor cells of the three groups of cancer patients. Significant associations were found between CCL2 & CCL5 and TNFα & IL-1β in the tumor cells in DCIS and IDC-no-relapse patients. In the IDC-with-relapse group, the expression of CCL2 & CCL5 was accompanied by further elevated incidence of TNFα & IL-1β expression. These results suggest progression-related roles for TNFα and IL-1β in breast cancer, as indeed indicated by the following: (1) Tumors of the IDC-with-relapse group had significantly higher persistence of TNFα and IL-1β compared to tumors of DCIS or IDC-no-relapse; (2) Continuous stimulation of the tumor cells by TNFα (and to some extent IL-1β) has led to EMT in the tumor cells; (3) Combined analyses with relevant clinical parameters suggested that IL-1β acts jointly with other pro-malignancy factors to promote disease relapse.
Conclusions: Our findings suggest that the coordinated expression of CCL2 & CCL5 and TNFα & IL-1β may be important for disease course, and that TNFα & IL-1β may promote disease relapse. Further in vitro and in vivo studies are needed for determination of the joint powers of the four factors in breast cancer, as well as analyses of their combined targeting in breast cancer.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

