Human NKT cells direct the differentiation of myeloid APCs that regulate T cell responses via expression of programmed cell death ligands
- PMID: 21486688
- PMCID: PMC3110565
- DOI: 10.1016/j.jaut.2011.03.001
Human NKT cells direct the differentiation of myeloid APCs that regulate T cell responses via expression of programmed cell death ligands
Abstract
NKT cells are innate lymphocytes that can recognize self or foreign lipids presented by CD1d molecules. NKT cells have been shown to inhibit the development of autoimmunity in murine model systems, however, the pathways by which they foster immune tolerance remain poorly understood. Here we show that autoreactive human NKT cells stimulate monocytes to differentiate into myeloid APCs that have a regulatory phenotype characterized by poor conjugate formation with T cells. The NKT cell instructed myeloid APCs show elevated expression of the inhibitory ligand PD-L2, and blocking PD-L1 and PD-L2 during interactions of the APCs with T cells results in improved cluster formation and significantly increased T cell proliferative responses. The elevated expression of PD-L molecules on NKT-instructed APCs appears to result from exposure to extracellular ATP that is produced during NKT-monocyte interactions, and blocking purinergic signaling during monocyte differentiation results in APCs that form clusters with T cells and stimulate their proliferation. Finally, we show that human monocytes and NKT cells that are injected into immunodeficient mice co-localize together in spleen and liver, and after 3 days in vivo in the presence of NKT cells a fraction of the myeloid cells have upregulated markers associated with differentiation into professional APCs. These results suggest that autoreactive human NKT cells may promote tolerance by inducing the differentiation of regulatory myeloid APCs that limit T cell proliferation through expression of PD-L molecules.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures
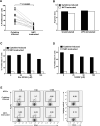
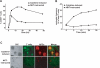
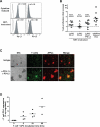
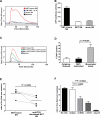

Similar articles
-
Autoreactive natural killer T cells: promoting immune protection and immune tolerance through varied interactions with myeloid antigen-presenting cells.Immunology. 2010 Aug;130(4):471-83. doi: 10.1111/j.1365-2567.2010.03293.x. Epub 2010 May 11. Immunology. 2010. PMID: 20465577 Free PMC article. Review.
-
Immunosuppressive myeloid-derived suppressor cells can be converted into immunogenic APCs with the help of activated NKT cells: an alternative cell-based antitumor vaccine.J Immunol. 2009 Feb 15;182(4):1818-28. doi: 10.4049/jimmunol.0802430. J Immunol. 2009. PMID: 19201833
-
Human NKT cells promote monocyte differentiation into suppressive myeloid antigen-presenting cells.J Leukoc Biol. 2009 Oct;86(4):757-68. doi: 10.1189/jlb.0209059. J Leukoc Biol. 2009. PMID: 19465641 Free PMC article.
-
PD-1 on immature and PD-1 ligands on migratory human Langerhans cells regulate antigen-presenting cell activity.J Invest Dermatol. 2010 Sep;130(9):2222-30. doi: 10.1038/jid.2010.127. Epub 2010 May 6. J Invest Dermatol. 2010. PMID: 20445553 Free PMC article.
-
The Role of Adaptor Proteins in the Biology of Natural Killer T (NKT) Cells.Front Immunol. 2019 Jun 25;10:1449. doi: 10.3389/fimmu.2019.01449. eCollection 2019. Front Immunol. 2019. PMID: 31293596 Free PMC article. Review.
Cited by
-
The Immune Landscape in Nonalcoholic Steatohepatitis.Immune Netw. 2016 Jun;16(3):147-58. doi: 10.4110/in.2016.16.3.147. Epub 2016 Jun 17. Immune Netw. 2016. PMID: 27340383 Free PMC article. Review.
-
The Pathophysiological Relevance of the iNKT Cell/Mononuclear Phagocyte Crosstalk in Tissues.Front Immunol. 2018 Oct 12;9:2375. doi: 10.3389/fimmu.2018.02375. eCollection 2018. Front Immunol. 2018. PMID: 30369933 Free PMC article. Review.
-
Invariant NKT cells as novel targets for immunotherapy in solid tumors.Clin Dev Immunol. 2012;2012:720803. doi: 10.1155/2012/720803. Epub 2012 Oct 17. Clin Dev Immunol. 2012. PMID: 23118781 Free PMC article. Review.
-
iNKT cells coordinate immune pathways to enable engraftment in nonconditioned hosts.Life Sci Alliance. 2021 Jun 10;4(7):e202000999. doi: 10.26508/lsa.202000999. Print 2021 Jul. Life Sci Alliance. 2021. PMID: 34112724 Free PMC article.
-
O death where is thy sting? Immunologic tolerance to apoptotic self.Cell Mol Life Sci. 2013 Oct;70(19):3571-89. doi: 10.1007/s00018-013-1261-0. Epub 2013 Feb 3. Cell Mol Life Sci. 2013. PMID: 23377225 Free PMC article. Review.
References
-
- Spada FM, Borriello F, Sugita M, Watts GF, Koezuka Y, Porcelli SA. Low expression level but potent antigen presenting function of CD1d on monocyte lineage cells. Eur. J. Immunol. 2000;30:3468–77. - PubMed
-
- Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, Ueno H, Nakagawa R, Sato H, Kondo E, et al. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science. 1997;278:1626–9. - PubMed
-
- Kinjo Y, Tupin E, Wu D, Fujio M, Garcia-Navarro R, Benhnia MR, Zajonc DM, Ben-Menachem G, Ainge GD, Painter GF, et al. Natural killer T cells recognize diacylglycerol antigens from pathogenic bacteria. Nat Immunol. 2006;7:978–86. - PubMed
-
- Kinjo Y, Wu D, Kim G, Xing GW, Poles MA, Ho DD, Tsuji M, Kawahara K, Wong CH, Kronenberg M. Recognition of bacterial glycosphingolipids by natural killer T cells. Nature. 2005;434:520–5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

