Excitatory-inhibitory imbalance in hypoglossal neurons during the critical period of postnatal development in the rat
- PMID: 21486774
- PMCID: PMC3090599
- DOI: 10.1113/jphysiol.2010.198945
Excitatory-inhibitory imbalance in hypoglossal neurons during the critical period of postnatal development in the rat
Abstract
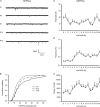
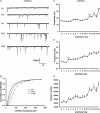
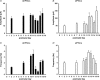
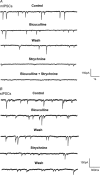
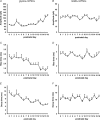
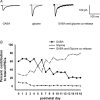
Hypoglossal motoneurons (HMs) innervate tongue muscles and are critical in maintaining patency of the upper airway during respiration. Abnormalities in HMs have been implicated in sudden infant death syndrome (SIDS) and obstructive sleep apnoea. Previously, we found a critical period in respiratory network development in rats around postnatal day (P) 12-13, when abrupt neurochemical, metabolic and physiological changes occurred. To test our hypothesis that an imbalance between inhibitory and excitatory synaptic transmission exists during the critical period, whole-cell patch-clamp recordings of HMs were done in brainstem slices of rats daily from P0 to P16. The results indicated that: (1) the amplitude and charge transfer of miniature excitatory postsynaptic currents (mEPSCs) were significantly reduced at P12-13; (2) the amplitude, mean frequency and charge transfer of miniature inhibitory postsynaptic currents (mIPSCs) were significantly increased at P12-13; (3) the kinetics (rise time and decay time) of both mEPSCs and mIPSCs accelerated with age; (4) the amplitude and frequency of spontaneous EPSCs were significantly reduced at P12-13, whereas those of spontaneous IPSCs were significantly increased at P12-13; and (5) both glycine and GABA contributed to mIPSCs. However, GABAergic currents fluctuated within a narrow range during the first three postnatal weeks, whereas glycinergic ones exhibited age-dependent changes comparable to those of total mIPSCs, indicating a reversal in dominance from GABA to glycine with development. Thus, our results provide strong electrophysiological evidence for an excitatory-inhibitory imbalance in HMs during the critical period of postnatal development in rats that may have significant implications for SIDS.
Figures
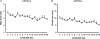
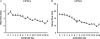
References
-
- Aldes LD, Chapman ME, Chronister RB, Haycock JW. Sources of noradrenergic afferents to the hypoglossal nucleus in the rat. Brain Res Bull. 1992;29:931–942. - PubMed
-
- Ballanyi K. Neuromodulation of the perinatal respiratory network. Curr Neuropharm. 2004;2:221–243. - PubMed
-
- Bekkers JM, Stevens CF. Presynaptic mechanism for long-term potentiation in the hippocampus. Nature. 1990;346:724–729. - PubMed
-
- Berger AJ, Bayliss DA, Viana F. Development of hypoglossal motoneurons. J Appl Physiol. 1996;81:1039–1048. - PubMed
-
- Burgard EC, Hablitz JJ. NMDA receptor-mediated components of miniature excitatory synaptic currents in developing rat neocortex. J Neurophysiol. 1993;70:1841–1852. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

