Lymphatic anatomy and biomechanics
- PMID: 21486777
- PMCID: PMC3139076
- DOI: 10.1113/jphysiol.2011.206672
Lymphatic anatomy and biomechanics
Abstract
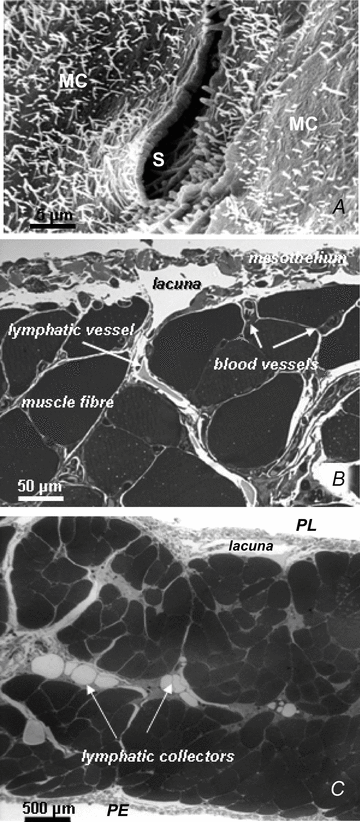
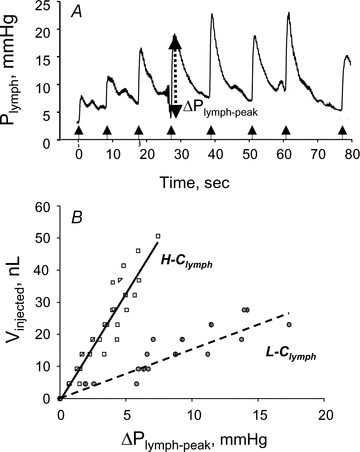
Lymph formation is driven by hydraulic pressure gradients developing between the interstitial tissue and the lumen of initial lymphatics. While in vessels equipped with lymphatic smooth muscle cells these gradients are determined by well-synchronized spontaneous contractions of vessel segments, initial lymphatics devoid of smooth muscles rely on tissue motion to form lymph and propel it along the network. Lymphatics supplying highly moving tissues, such as skeletal muscle, diaphragm or thoracic tissues, undergo cyclic compression and expansion of their lumen imposed by local stresses arising in the tissue as a consequence of cardiac and respiratory activities. Active muscle contraction and not passive tissue displacement is required to support an efficient lymphatic drainage, as suggested by the fact that the respiratory activity promotes lymph formation during spontaneous, but not mechanical ventilation. The mechanical properties of the lymphatic wall and of the surrounding tissue also play an important role in lymphatic function. Modelling of stress distribution in the lymphatic wall suggests that compliant vessels behave as reservoirs accommodating absorbed interstitial fluid, while lymphatics with stiffer walls, taking advantage of a more efficient transmission of tissue stresses to the lymphatic lumen, propel fluid through the lumen of the lymphatic circuit.
Figures



References
-
- Aukland K, Reed R. Interstitial–lymphatic mechanisms in the control of extracellular fluid volume. Physiol Rev. 1993;73:1–78. - PubMed
-
- Benoit JN, Zawieja DC, Goodman AH, Granger HJ. Characterization of intact mesenteric lymphatic pump and its responsiveness to acute edemagenic stress. Am J Physiol Heart Circ Physiol. 1989;257:H2059–H2069. - PubMed
-
- Burgeson RE, Lunstrum GP, Rokosova B, Rimberg CS, Rosenbaum LM, Keene DR. The structure and function of type VII collagen. Ann N Y Acad Sci. 1990;580:32–43. - PubMed
-
- Casley-Smith JR, Florey HW. The structure of normal small lymphatics. Q J Exp Physiol Cogn Med Sci. 1961;46:101–106. - PubMed
-
- Castenholz A. Structural and functional properties of initial lymphatics in the rat tongue: scanning electron microscopic findings. Lymphology. 1987;20:112–125. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

