Volume regulation in mammalian skeletal muscle: the role of sodium-potassium-chloride cotransporters during exposure to hypertonic solutions
- PMID: 21486779
- PMCID: PMC3112562
- DOI: 10.1113/jphysiol.2011.206730
Volume regulation in mammalian skeletal muscle: the role of sodium-potassium-chloride cotransporters during exposure to hypertonic solutions
Abstract
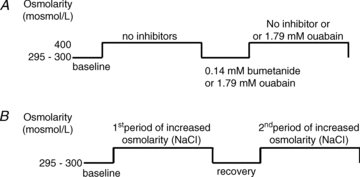
Controversy exists as to whether mammalian skeletal muscle is capable of volume regulation in response to changes in extracellular osmolarity despite evidence that muscle fibres have the required ion transport mechanisms to transport solute and water in situ. We addressed this issue by studying the ability of skeletal muscle to regulate volume during periods of induced hyperosmotic stress using single, mouse extensor digitorum longus (EDL) muscle fibres and intact muscle (soleus and EDL). Fibres and intact muscles were loaded with the fluorophore, calcein, and the change in muscle fluorescence and width (single fibres only) used as a metric of volume change. We hypothesized that skeletal muscle exposed to increased extracellular osmolarity would elicit initial cellular shrinkage followed by a regulatory volume increase (RVI) with the RVI dependent on the sodium–potassium–chloride cotransporter (NKCC). We found that single fibres exposed to a 35% increase in extracellular osmolarity demonstrated a rapid, initial 27–32% decrease in cell volume followed by a RVI which took 10-20 min and returned cell volume to 90–110% of pre-stimulus values. Within intact muscle, exposure to increased extracellular osmolarity of varying degrees also induced a rapid, initial shrinkage followed by a gradual RVI, with a greater rate of initial cell shrinkage and a longer time for RVI to occur with increasing extracellular tonicities. Furthermore, RVI was significantly faster in slow-twitch soleus than fast-twitch EDL. Pre-treatment of muscle with bumetanide (NKCC inhibitor) or ouabain (Na+,K+-ATPase inhibitor), increased the initial volume loss and impaired the RVI response to increased extracellular osmolarity indicating that the NKCC is a primary contributor to volume regulation in skeletal muscle. It is concluded that mouse skeletal muscle initially loses volume then exhibits a RVI when exposed to increases in extracellular osmolarity. The rate of RVI is dependent on the degree of change in extracellular osmolarity, is muscle specific, and is dependent on the functioning of the NKCC and Na+, K+-ATPase.
Figures


Comment in
-
Dimethyl sulphoxide addition or withdrawal causes biphasic volume changes and its withdrawal causes t-system vacuolation in skeletal muscle.J Physiol. 2011 Nov 15;589(Pt 22):5555-6; author reply 5557. doi: 10.1113/jphysiol.2011.218313. J Physiol. 2011. PMID: 22086252 Free PMC article. No abstract available.
References
-
- Antolic A, Harrison R, Farlinger C, Cermak NM, Peters SJ, LeBlanc P, Roy BD. The effect of ectracellular osmolarity on cell volume and resting metabolism in mammalian skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1994–R2000. - PubMed
-
- Bressler BH. Isometric tension and instantaneous stiffness in amphibian skeletal muscle exposed to solutions of increased osmolarity. Can J Physiol Pharmacol. 1977;55:1208–1210. - PubMed
-
- Cermak NM, LeBlanc PJ, Peters SJ, Vandenboom R, Roy BD. Effect of extracellular osmolality on metabolism in contracting mammalian skeletal muscle in vitro. Appl Physiol Nutr Metab. 2009;34:1055–1064. - PubMed
-
- Crowe WE, Altamirano J, Huerto L, Alvarez-Leefmans FJ. Volume changes in single N1E-115 neuroblastoma cells measured with a fluorescent probe. Neuroscience. 1995;69:283–296. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

