Axonal failure during high frequency stimulation of rat subthalamic nucleus
- PMID: 21486784
- PMCID: PMC3112555
- DOI: 10.1113/jphysiol.2011.205807
Axonal failure during high frequency stimulation of rat subthalamic nucleus
Abstract
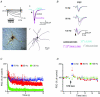
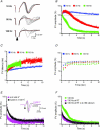
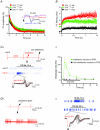
Deep brain stimulation (DBS) has been established as an effective surgical therapy for advanced Parkinson's disease (PD) and gains increasing acceptance for otherwise intractable neuropsychiatric diseases such as major depression or obsessive–compulsive disorders. In PD, DBS targets predominantly the subthalamic nucleus (STN) and relieves motor deficits only at high frequency (>100 Hz). In contrast to the well-documented clinical efficacy of DBS, its underlying principle remains enigmatic spawning a broad and, in part, contradictory spectrum of suggested synaptic and non-synaptic mechanisms within and outside STN. Here we focused on a crucial, but largely neglected issue in this controversy, namely the axonal propagation of DBS within and away from STN. In rat brain slices preserving STN projections to substantia nigra (SN) and entopeduncular nucleus (EP, the rodent equivalent of internal globus pallidus), STN-DBS disrupted synaptic excitation onto target neurons through an unexpected failure of axonal signalling. The rapid onset and, upon termination of DBS, recovery of this effect was highly reminiscent of the time course of DBS in the clinical setting. We propose that DBS-induced suppression of axonal projections from and to STN serves to shield basal ganglia circuitry from pathological activity arising in or amplified by this nucleus.
Figures

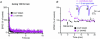

References
-
- Ammari R, Lopez C, Bioulac B, Garcia L, Hammond C. Subthalamic nucleus evokes similar long lasting glutamatergic excitations in pallidal, entopeduncular and nigral neurons in the basal ganglia slice. Neuroscience. 2010;166:808–818. - PubMed
-
- Ammari R, Lopez C, Fiorentino H, Gonon F, Hammond C. A mouse juvenile or adult slice with preserved functional nigro-striatal dopaminergic neurons. Neuroscience. 2009;159:3–6. - PubMed
-
- Bekar L, Libionka W, Tian GF, Xu Q, Torres A, Wang X, Lovatt D, Williams E, Takano T, Schnermann J, Bakos R, Nedergaard M. Adenosine is crucial for deep brain stimulation-mediated attenuation of tremor. Nat Med. 2008;14:75–80. - PubMed
-
- Bellinger SC, Miyazawa G, Steinmetz PN. Submyelin potassium accumulation may functionally block subsets of local axons during deep brain stimulation: a modeling study. J Neural Eng. 2008;5:263–274. - PubMed
-
- Beurrier C, Ben-Ari Y, Hammond C. Preservation of the direct and indirect pathways in an in vitro preparation of the mouse basal ganglia. Neuroscience. 2006;140:77–86. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

