Transmission of colour and acuity signals by parvocellular cells in marmoset monkeys
- PMID: 21486786
- PMCID: PMC3112556
- DOI: 10.1113/jphysiol.2010.194076
Transmission of colour and acuity signals by parvocellular cells in marmoset monkeys
Abstract
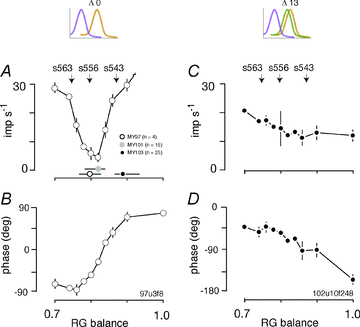
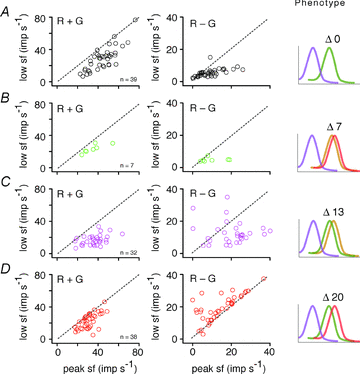
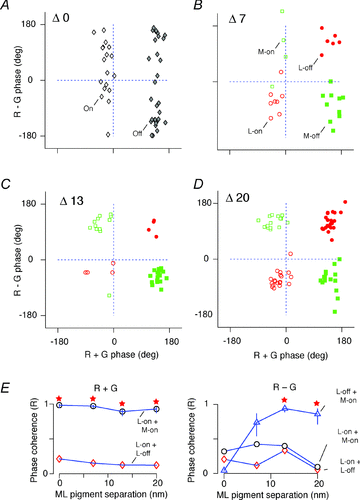
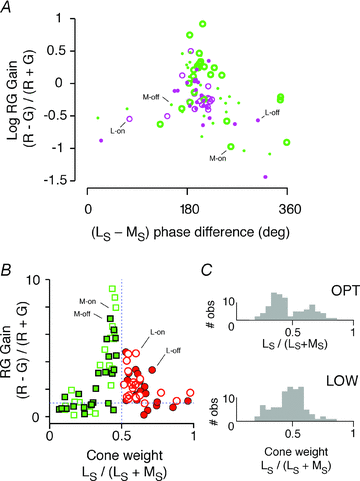
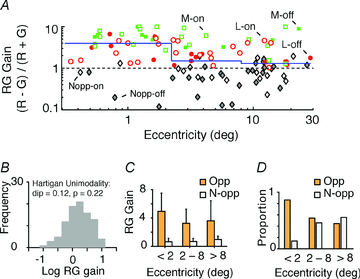
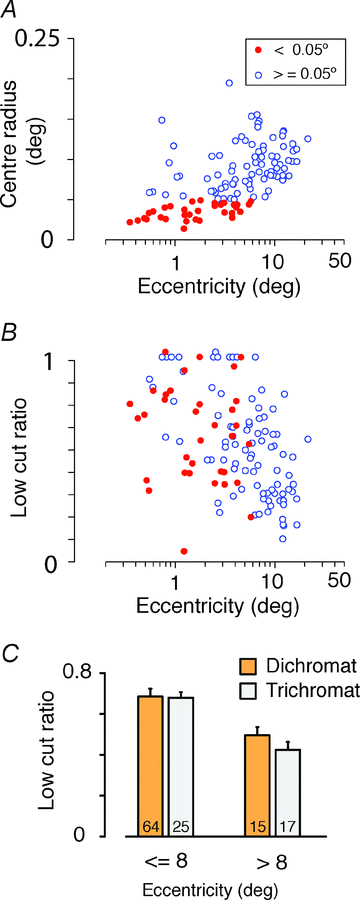
The red-green axis of colour vision evolved recently in primate evolutionary history. Signals serving red-green colour vision travel together with signals serving spatial vision, in the parvocellular (PC) division of the subcortical visual pathway. However, the question of whether receptive fields of PC pathway cells are specialized to transmit red-green colour signals remains unresolved. We addressed this question in single-cell recordings from the lateral geniculate nucleus of anaesthetized marmosets. Marmosets show a high proportion of dichromatic (red-green colour-blind) individuals, allowing spatial and colour tuning properties of PC cells to be directly compared in dichromatic and trichromatic visual systems. We measured spatial frequency tuning for sine gratings that provided selective stimulation of individual photoreceptor types. We found that in trichromatic marmosets, the foveal visual field representation is dominated by red-green colour-selective PC cells. Colour selectivity of PC cells is reduced at greater eccentricities, but cone inputs to centre and surround are biased to create more selectivity than predicted by a purely 'random wiring' model. Thus, one-to-one connections in the fovea are sufficient, but not necessary, to create colour-selective responses. The distribution of spatial tuning properties for achromatic stimuli shows almost complete overlap between PC cells recorded in dichromatic and trichromatic marmosets. These data indicate that transmission of red-green colour signals has been enabled by centre-surround receptive fields of PC cells, and has not altered the capacity of PC cells to serve high-acuity vision at high stimulus contrast.
Figures

References
-
- Bowmaker JK, Jacobs GH, Mollon JD. Polymorphism of photopigments in the squirrel monkey: a sixth phenotype. Proc R Soc Lond B Biol Sci. 1987;231:383–390. - PubMed
-
- Boycott BB, Wässle H. Morphological classification of bipolar cells of the primate retina. Eur J Neurosci. 1991;3:1069–1088. - PubMed
-
- Brainard DH. Cone contrast and opponent modulation color spaces. In: Kaiser PK, Boynton GM, editors. Human Color Vision. Washington, DC: Optical Society of America; 1996. pp. 563–577.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

