Structural heterogeneity promotes triggered activity, reflection and arrhythmogenesis in cardiomyocyte monolayers
- PMID: 21486795
- PMCID: PMC3098708
- DOI: 10.1113/jphysiol.2010.200576
Structural heterogeneity promotes triggered activity, reflection and arrhythmogenesis in cardiomyocyte monolayers
Abstract
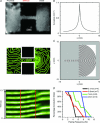
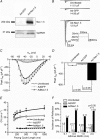
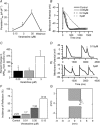
Patients with structural heart disease are predisposed to arrhythmias by incompletely understood mechanisms. We hypothesized that tissue expansions promote source-to-sink mismatch leading to early after-depolarizations (EADs) and reflection of impulses in monolayers of well-polarized neonatal rat ventricular cardiomyocytes.We traced electrical propagation optically in patterned monolayers consisting of two wide regions connected by a thin isthmus.Structural heterogeneities provided a substrate for EADs, retrograde propagation along the same pathway (reflection) and reentry initiation. Reflection always originated during the action potential (AP) plateau at the distal expansion. To determine whether increased sodium current(INa) would promote EADs, we employed adenoviral transfer of Nav1.5 (Ad-Nav1.5). Compared with uninfected and adenoviral expression of green fluorescent protein (Ad-GFP; viral control),Ad-Nav1.5 significantly increased Nav1.5 protein expression, peak and persistent INa density, A Pupstroke velocity, AP duration, conduction velocity and EAD incidence, as well as reflection incidence (29.2%, n =48 vs. uninfected, 9.4%, n =64; and Ad-GFP, 4.8%, n =21). Likewise,the persistent INa agonist veratridine (0.05–3 μM) prolonged the AP, leading to EADs and reflection. Reflection led to functional reentry distally and bigeminal and trigeminal rhythms proximally. Reflection was rare in the absence of structural heterogeneities.Computer simulations demonstrated the importance of persistent INa in triggering reflection and predicted that the gradient between the depolarizing cells at the distal expansion and the repolarizing cells within the isthmus enabled retrograde flow of depolarizing electrotonic current to trigger EADs and reflection. A combination of a substrate (structural heterogeneity) and a trigger (increased persistent INa and EADs) promotes reflection and arrhythmogenesis.
Figures




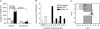



Comment in
-
Expanding on forty years of reflection.J Physiol. 2011 May 1;589(Pt 9):2107-8. doi: 10.1113/jphysiol.2011.209239. J Physiol. 2011. PMID: 21532028 Free PMC article. No abstract available.
Similar articles
-
Expanding on forty years of reflection.J Physiol. 2011 May 1;589(Pt 9):2107-8. doi: 10.1113/jphysiol.2011.209239. J Physiol. 2011. PMID: 21532028 Free PMC article. No abstract available.
-
Slow [Na]i Changes and Positive Feedback Between Membrane Potential and [Ca]i Underlie Intermittent Early Afterdepolarizations and Arrhythmias.Circ Arrhythm Electrophysiol. 2015 Dec;8(6):1472-80. doi: 10.1161/CIRCEP.115.003085. Epub 2015 Sep 25. Circ Arrhythm Electrophysiol. 2015. PMID: 26407967 Free PMC article.
-
Role of the transient outward potassium current in the genesis of early afterdepolarizations in cardiac cells.Cardiovasc Res. 2012 Aug 1;95(3):308-16. doi: 10.1093/cvr/cvs183. Epub 2012 Jun 1. Cardiovasc Res. 2012. PMID: 22660482 Free PMC article.
-
Early afterdepolarizations in cardiac myocytes: beyond reduced repolarization reserve.Cardiovasc Res. 2013 Jul 1;99(1):6-15. doi: 10.1093/cvr/cvt104. Epub 2013 Apr 25. Cardiovasc Res. 2013. PMID: 23619423 Free PMC article. Review.
-
The arrhythmogenic consequences of increasing late INa in the cardiomyocyte.Cardiovasc Res. 2013 Sep 1;99(4):600-11. doi: 10.1093/cvr/cvt145. Epub 2013 Jun 10. Cardiovasc Res. 2013. PMID: 23752976 Free PMC article. Review.
Cited by
-
Mathematical Analysis of the Role of Heterogeneous Distribution of Excitable and Non-excitable Cells on Early Afterdepolarizations.Front Phys. 2018;6:10.3389/fphy.2018.00117. doi: 10.3389/fphy.2018.00117. Epub 2018 Oct 12. Front Phys. 2018. PMID: 40909466 Free PMC article.
-
Cardiac autonomic nerve distribution and arrhythmia.Neural Regen Res. 2012 Dec 15;7(35):2834-41. doi: 10.3969/j.issn.1673-5374.2012.35.012. Neural Regen Res. 2012. PMID: 25317134 Free PMC article.
-
New methods for the analysis of heartbeat behavior in risk stratification.Front Physiol. 2011 Nov 29;2:88. doi: 10.3389/fphys.2011.00088. eCollection 2011. Front Physiol. 2011. PMID: 22144963 Free PMC article.
-
Molecular and genetic basis of sudden cardiac death.J Clin Invest. 2013 Jan;123(1):75-83. doi: 10.1172/JCI62928. Epub 2013 Jan 2. J Clin Invest. 2013. PMID: 23281413 Free PMC article. Review.
-
Expanding on forty years of reflection.J Physiol. 2011 May 1;589(Pt 9):2107-8. doi: 10.1113/jphysiol.2011.209239. J Physiol. 2011. PMID: 21532028 Free PMC article. No abstract available.
References
-
- Antzelevitch C. Electrotonic modulation of conduction and automaticity. In: Janse H, Meijler F, Van Der Tweet L, editors. Proceedings of the Royal Academy of Arts and Sciences of the Netherlands. North-Holland, Amsterdam, The Netherlands: 1990.
-
- Antzelevitch C, Jalife J, Moe GK. Characteristics of reflection as a mechanism of reentrant arrhythmias and its relationship to parasystole. Circulation. 1980;61:182–191. - PubMed
-
- Arnestad M, Crotti L, Rognum TO, Insolia R, Pedrazzini M, Ferrandi C, Vege A, Wang DW, Rhodes TE, George AL, Jr, Schwartz PJ. Prevalence of long-QT syndrome gene variants in sudden infant death syndrome. Circulation. 2007;115:361–367. - PubMed
-
- Cabo C, Barr RC. Propagation model using the DiFrancesco-Noble equations. Comparison to reported experimental results. Med Biol Eng Comput. 1992;30:292–302. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

