Mitochondrial production of reactive oxygen species contributes to the β-adrenergic stimulation of mouse cardiomycytes
- PMID: 21486840
- PMCID: PMC3099030
- DOI: 10.1113/jphysiol.2010.202838
Mitochondrial production of reactive oxygen species contributes to the β-adrenergic stimulation of mouse cardiomycytes
Abstract
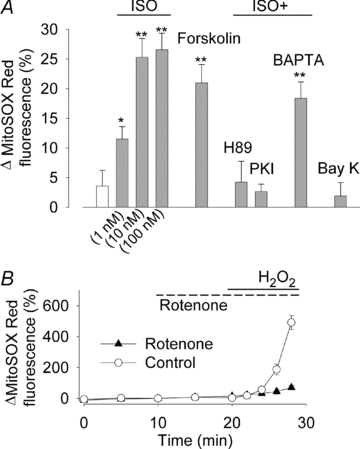
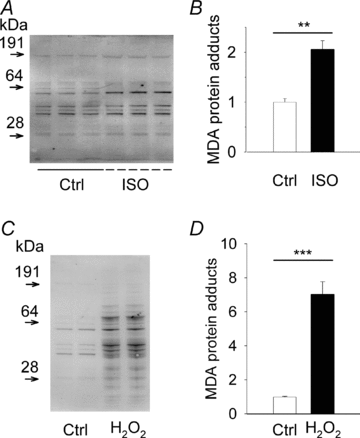
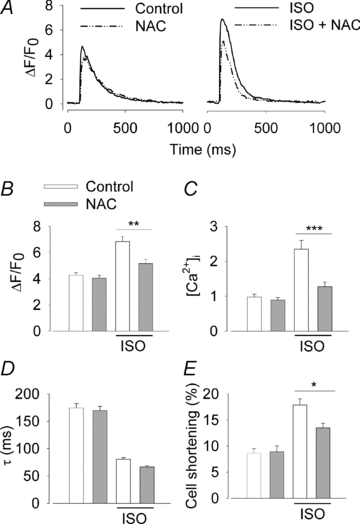
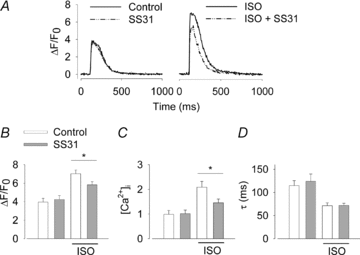
The sympathetic adrenergic system plays a central role in stress signalling and stress is often associated with increased production of reactive oxygen species (ROS). Furthermore, the sympathetic adrenergic system is intimately involved in the regulation of cardiomyocyte Ca2+ handling and contractility. In this study we hypothesize that endogenously produced ROS contribute to the inotropic mechanism of β-adrenergic stimulation in mouse cardiomyocytes. Cytoplasmic Ca2+ transients, cell shortening and ROS production were measured in freshly isolated cardiomyocytes using confocal microscopy and fluorescent indicators. As a marker of oxidative stress, malondialdehyde (MDA) modification of proteins was detected with Western blotting. Isoproterenol (ISO), a β-adrenergic agonist, increased mitochondrial ROS production in cardiomyocytes in a concentration- and cAMP–protein kinase A-dependent but Ca2+-independent manner. Hearts perfused with ISO showed a twofold increase in MDA protein adducts relative to control. ISO increased Ca2+ transient amplitude, contraction and L-type Ca2+ current densities (measured with whole-cell patch-clamp) in cardiomyocytes and these increases were diminished by application of the general antioxidant N-acetylcysteine (NAC) or the mitochondria-targeted antioxidant SS31. In conclusion, increased mitochondrial ROS production plays an integral role in the acute inotropic response of cardiomyocytes to β-adrenergic stimulation. On the other hand, chronically sustained adrenergic stress is associated with the development of heart failure and cardiac arrhythmias and prolonged increases in ROS may contribute to these defects.
Figures


References
-
- Aldini G, Dalle-Donne I, Facino RM, Milzani A, Carini M. Intervention strategies to inhibit protein carbonylation by lipoxidation-derived reactive carbonyls. Med Res Rev. 2007;27:817–868. - PubMed
-
- Andrade FH, Reid MB, Westerblad H. Contractile response of skeletal muscle to low peroxide concentrations: myofibrillar calcium sensitivity as a likely target for redox-modulation. FASEB J. 2001;15:309–311. - PubMed
-
- Aydin J, Andersson DC, Hänninen SL, Wredenberg A, Tavi P, Park CB, Larsson NG, Bruton JD, Westerblad H. Increased mitochondrial Ca2+ and decreased sarcoplasmic reticulum Ca2+ in mitochondrial myopathy. Human Mol Genet. 2009;18:278–288. - PubMed
-
- Bassani JW, Yuan W, Bers DM. Fractional SR Ca release is regulated by trigger Ca and SR Ca content in cardiac myocytes. Am J Physiol Cell Physiol. 1995;268:C1313–C1319. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

