Impact of the use of autologous stem cell transplantation at first relapse both in naive and previously rituximab exposed follicular lymphoma patients treated in the GELA/GOELAMS FL2000 study
- PMID: 21486862
- PMCID: PMC3148906
- DOI: 10.3324/haematol.2010.030320
Impact of the use of autologous stem cell transplantation at first relapse both in naive and previously rituximab exposed follicular lymphoma patients treated in the GELA/GOELAMS FL2000 study
Abstract
Background: We analyzed detailed characteristics and salvage treatment in 175 follicular lymphoma patients from the FL2000 study who were in progression after first-line therapy with or without addition of rituximab to chemotherapy and interferon.
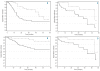
Design and methods: The impact of using autologous stem cell transplantation and/or rituximab administration at first progression was investigated, taking into account initial therapy. With a median follow up of 31 months, 3-year event free and overall survival rates after progression were 50% (95%CI 42-58%) and 72% (95%CI 64-78%), respectively.
Results: The 3-year event free rate of rituximab re-treated patients (n=112) was 52% (95%CI 41-62%) versus 40% (95%CI 24-55%) for those not receiving rituximab second line (n=53) (P=0.075). There was a significant difference in 3-year overall survival between patients receiving autologous stem cell transplantation and those not: 92% (95%CI 78-97%) versus 63% (95%CI 51-72%) (P=0.0003), respectively. In multivariate analysis, both autologous stem cell transplantation and period of progression/relapse affected event free and overall survival.
Conclusions: Regardless of front-line rituximab exposure, this study supports incorporating autologous stem cell transplantation in the therapeutic approach at first relapse for follicular lymphoma patients.
Figures



References
-
- Liu Q, Fayad L, Cabanillas F, Hagemeister FB, Ayers GD, Hess M, et al. Improvement of overall and failure-free survival in stage IV follicular lymphoma: 25 years of treatment experience at The University of Texas M.D. Anderson Cancer Center. J Clin Oncol. 2006;24(10):1582–9. - PubMed
-
- Pulte D, Gondos A, Brenner H. Ongoing improvement in outcomes for patients diagnosed as having Non-Hodgkin lymphoma from the 1990s to the early 21st century. Arch Intern Med. 2008;168(5):469–76. - PubMed
-
- Herold M, Haas A, Srock S, Neser S, Al-Ali KH, Neubauer A, et al. East German Study Group Hematology and Oncology Study. Rituximab added to first-line mitoxantrone, chlorambucil, and prednisolone chemotherapy followed by interferon maintenance prolongs survival in patients with advanced follicular lymphoma: an East German Study Group Hematology and Oncology Study. J Clin Oncol. 2007;25(15):1986–92. - PubMed
-
- Hiddemann W, Kneba M, Dreyling M, Schmitz N, Lengfelder E, Schmits R, et al. Frontline therapy with rituximab added to the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) significantly improves the outcome for patients with advanced-stage follicular lymphoma compared with therapy with CHOP alone: results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood. 2005;106(12):3725–32. - PubMed
-
- Marcus R, Imrie K, Solal-Celigny P, Catalano JV, Dmoszynska A, Raposo JC, et al. Phase III study of R-CVP compared with cyclophosphamide, vincristine, and prednisone alone in patients with previously untreated advanced follicular lymphoma. J Clin Oncol. 2008;26(28):4579–86. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

