Small RNAs in early mammalian development: from gametes to gastrulation
- PMID: 21486922
- PMCID: PMC3074443
- DOI: 10.1242/dev.056234
Small RNAs in early mammalian development: from gametes to gastrulation
Abstract
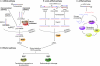
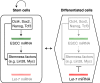
Small non-coding RNAs, including microRNAs (miRNAs), endogenous small interfering RNAs (endo-siRNAs) and Piwi-interacting RNAs (piRNAs), play essential roles in mammalian development. The function and timing of expression of these three classes of small RNAs differ greatly. piRNAs are expressed and play a crucial role during male gametogenesis, whereas endo-siRNAs are essential for oocyte meiosis. By contrast, miRNAs are ubiquitously expressed in somatic tissues and function throughout post-implantation development. Surprisingly, however, miRNAs are non-essential during pre-implantation embryonic development and their function is suppressed during oocyte meiosis. Here, we review the roles of small non-coding RNAs during the early stages of mammalian development, from gamete maturation through to gastrulation.
Figures

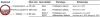
References
-
- Abe K., Inoue A., Suzuki M. G., Aoki F. (2010). Global gene silencing is caused by the dissociation of RNA polymerase II from DNA in mouse oocytes. J. Reprod. Dev. 56, 502-507 - PubMed
-
- Amabile G., Meissner A. (2009). Induced pluripotent stem cells: current progress and potential for regenerative medicine. Trends Mol. Med. 15, 59-68 - PubMed
-
- Aravin A., Gaidatzis D., Pfeffer S., Lagos-Quintana M., Landgraf P., Iovino N., Morris P., Brownstein M. J., Kuramochi-Miyagawa S., Nakano T., et al. (2006). A novel class of small RNAs bind to MILI protein in mouse testes. Nature 442, 203-207 - PubMed
-
- Aravin A. A., Sachidanandam R., Girard A., Fejes-Toth K., Hannon G. J. (2007). Developmentally regulated piRNA clusters implicate MILI in transposon control. Science 316, 744-747 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

