Canonical Wnt signaling promotes the proliferation and neurogenesis of peripheral olfactory stem cells during postnatal development and adult regeneration
- PMID: 21486944
- PMCID: PMC3078819
- DOI: 10.1242/jcs.080580
Canonical Wnt signaling promotes the proliferation and neurogenesis of peripheral olfactory stem cells during postnatal development and adult regeneration
Abstract
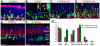
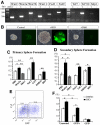
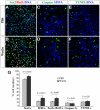
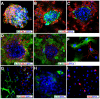
The mammalian olfactory epithelium (OE) has a unique stem cell or progenitor niche, which is responsible for the constant peripheral neurogenesis throughout the lifespan of the animal. However, neither the signals that regulate the behavior of these cells nor the lineage properties of the OE stem cells are well understood. Multiple Wnt signaling components exhibit dynamic expression patterns in the developing OE. We generated Wnt signaling reporter TOPeGFP transgenic mice and found TOPeGFP activation predominantly in proliferating Sox2(+) OE basal cells during early postnatal development. FACS-isolated TOPeGFP(+) OE basal cells are required, but are not sufficient, for formation of spheres. Wnt3a significantly promotes the proliferation of the Sox2(+) OE sphere cells. Wnt-stimulated OE sphere cells maintain their multipotency and can differentiate into most types of neuronal and non-neuronal epithelial cells. Also, Wnt activators shift the production of differentiated cells toward olfactory sensory neurons. Moreover, TOPeGFP(+) cells are robustly increased in the adult OE after injury. In vivo administration of Wnt modulators significantly alters the regeneration potential. This study demonstrates the role of the canonical Wnt signaling pathway in the regulation of OE stem cells or progenitors during development and regeneration.
Figures



References
-
- Bandyopadhyay U., Biswas K., Banerjee R. K. (2002). Extrathyroidal actions of antithyroid thionamides. Toxicol. Lett. 128, 117-127 - PubMed
-
- Barraud P., He X., Zhao C., Ibanez C., Raha-Chowdhury R., Caldwell M. A., Franklin R. J. (2007). Contrasting effects of basic fibroblast growth factor and epidermal growth factor on mouse neonatal olfactory mucosa cells. Eur. J. Neurosci. 26, 3345-3357 - PubMed
-
- Bergman U., Ostergren A., Gustafson A. L., Brittebo B. (2002). Differential effects of olfactory toxicants on olfactory regeneration. Arch. Toxicol. 76, 104-112 - PubMed
-
- Bergstrom U., Giovanetti A., Piras E., Brittebo E. B. (2003). Methimazole-induced damage in the olfactory mucosa: effects on ultrastructure and glutathione levels. Toxicol. Pathol. 31, 379-387 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

