Actomyosin-generated tension controls the molecular kinetics of focal adhesions
- PMID: 21486952
- PMCID: PMC3078811
- DOI: 10.1242/jcs.077388
Actomyosin-generated tension controls the molecular kinetics of focal adhesions
Abstract
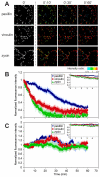
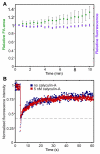
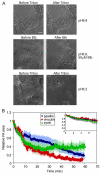
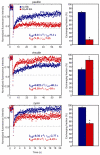
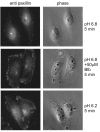
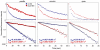
Focal adhesions (FAs) have key roles in the interaction of cells with the extracellular matrix (ECM) and in adhesion-mediated signaling. These dynamic, multi-protein structures sense the ECM both chemically and physically, and respond to external and internal forces by changing their size and signaling activity. However, this mechanosensitivity is still poorly understood at the molecular level. Here, we present direct evidence that actomyosin contractility regulates the molecular kinetics of FAs. We show that the molecular turnover of proteins within FAs is primarily regulated by their dissociation rate constant (k(off)), which is sensitive to changes in forces applied to the FA. We measured the early changes in k(off) values for three FA proteins (vinculin, paxillin and zyxin) upon inhibition of actomyosin-generated forces using two methods - high temporal resolution FRAP and direct measurement of FA protein dissociation in permeabilized cells. When myosin II contractility was inhibited, the k(off) values for all three proteins changed rapidly, in a highly protein-specific manner: dissociation of vinculin from FAs was facilitated, whereas dissociation of paxillin and zyxin was attenuated. We hypothesize that these early kinetic changes initiate FA disassembly by affecting the molecular turnover of FAs and altering their composition.
Figures

References
-
- Brown C. M., Hebert B., Kolin D. L., Zareno J., Whitmore L., Horwitz A. R., Wiseman P. W. (2006). Probing the integrin-actin linkage using high-resolution protein velocity mapping. J. Cell Sci. 119, 5204-5214 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

