Genital shedding of herpes simplex virus among symptomatic and asymptomatic persons with HSV-2 infection
- PMID: 21486977
- PMCID: PMC3144252
- DOI: 10.1001/jama.2011.420
Genital shedding of herpes simplex virus among symptomatic and asymptomatic persons with HSV-2 infection
Abstract
Context: Since herpes simplex virus type 2 (HSV-2) antibody tests have become commercially available, an increasing number of persons have learned that they have genital herpes through serologic testing. The course of natural history of HSV-2 in asymptomatic, seropositive persons is uncertain.
Objective: To evaluate the virologic and clinical course of HSV genital shedding among individuals with symptomatic and asymptomatic HSV-2 infection.
Design, setting, and participants: Cohort of 498 immunocompetent HSV-2-seropositive persons enrolled in prospective studies of genital HSV shedding at the University of Washington Virology Research Clinic, Seattle, and Westover Heights Clinic, Portland, Oregon, between March 1992 and April 2008. Each participant obtained daily self-collected swabs of genital secretions for at least 30 days.
Main outcome measure: The rate of viral shedding measured by quantitative real-time fluorescence polymerase chain reaction for HSV DNA from genital swabs.
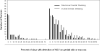
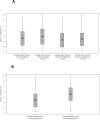
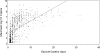
Results: Herpes simplex virus type 2 was detected on 4753 of 23,683 days (20.1%; 95% confidence interval [CI], 18.3%-22.0%) in 410 persons with symptomatic genital HSV-2 infection compared with 519 of 5070 days (10.2%; 95% CI, 7.7%-13.6%) in 88 persons with asymptomatic infection (P < .001). Subclinical shedding rates were higher in persons with symptomatic infection compared with asymptomatic infection (2708 of 20,735 days [13.1%; 95% CI, 11.5%-14.6%) vs 434 of 4929 days [8.8%; 95% CI, 6.3%-11.5%]) (P < .001). However, the amount of HSV detected during subclinical shedding episodes was similar (median, 4.3 [interquartile range, 3.1-5.6] log(10) copies in the symptomatic infection group vs 4.2 [interquartile range, 2.9-5.5] in the asymptomatic infection group, P = .27). Days with lesions accounted for 2045 of 4753 days (43.0%; 95% CI, 39.8%-46.5%) with genital viral shedding among persons with symptomatic genital HSV-2 infection compared with 85 of 519 days (16.4%; 95% CI, 11.2%-23.9%) among persons with asymptomatic infection (P < .001).
Conclusion: Persons with asymptomatic HSV-2 infection shed virus in the genital tract less frequently than persons with symptomatic infection, but much of the difference is attributable to less frequent genital lesions because lesions are accompanied by frequent viral shedding.
Figures
References
-
- Mertz KJ, Trees D, Levine WC, et al. Etiology of genital ulcers and prevalence of human immunodeficiency virus coinfection in 10 US cities. The Genital Ulcer Disease Surveillance Group. J Infect Dis. 1998 Dec;178(6):1795–1798. - PubMed
-
- Seroprevalence of herpes simplex virus type 2 among persons aged 14-49 years--United States, 2005-2008. MMWR Morb Mortal Wkly Rep. 2010 Apr 23;59(15):456–459. - PubMed
-
- Wald A, Zeh J, Selke S, et al. Reactivation of genital herpes simplex virus type 2 infection in asymptomatic seropositive persons. N Engl J Med. 2000 Mar 23;342(12):844–850. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

