A novel specificity protein 1 (SP1)-like gene regulating protein kinase C-1 (Pkc1)-dependent cell wall integrity and virulence factors in Cryptococcus neoformans
- PMID: 21487010
- PMCID: PMC3121451
- DOI: 10.1074/jbc.M111.230268
A novel specificity protein 1 (SP1)-like gene regulating protein kinase C-1 (Pkc1)-dependent cell wall integrity and virulence factors in Cryptococcus neoformans
Abstract
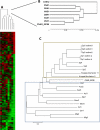
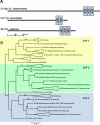
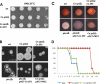
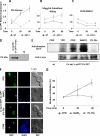
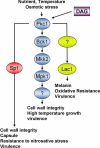
Eukaryotic cells utilize complex signaling systems to detect their environments, responding and adapting as new conditions arise during evolution. The basidiomycete fungus Cryptococcus neoformans is a leading cause of AIDS-related death worldwide and utilizes the calcineurin and protein kinase C-1 (Pkc1) signaling pathways for host adaptation and expression of virulence. In the present studies, a C-terminal zinc finger transcription factor, homologous both to the calcineurin-responsive zinc fingers (Crz1) of ascomycetes and to the Pkc1-dependent specificity protein-1 (Sp1) transcription factors of metazoans, was identified and named SP1 because of its greater similarity to the metazoan factors. Structurally, the Cryptococcus neoformans Sp1 (Cn Sp1) protein was found to have acquired an additional zinc finger motif from that of Crz1 and showed Pkc1-dependent phosphorylation, nuclear localization, and whole genome epistatic associations under starvation conditions. Transcriptional targets of Cn Sp1 shared functional similarities with Crz1 factors, such as cell wall synthesis, but gained the regulation of processes involved in carbohydrate metabolism, including trehalose metabolism, and lost others, such as the induction of autophagy. In addition, overexpression of Cn Sp1 in a pkc1Δ mutant showed restoration of altered phenotypes involved in virulence, including cell wall stability, nitrosative stress, and extracellular capsule production. Cn Sp1 was also found to be important for virulence of the fungus using a mouse model. In summary, these data suggest an evolutionary shift in C-terminal zinc finger proteins during fungal evolution, transforming them from calcineurin-dependent to PKC1-dependent transcription factors, helping to shape the role of fungal pathogenesis of C. neoformans.
Figures

References
-
- Bichile L. S., Gokhale Y. A., Sridhar V., Gill N. H. (2001) J. Assoc. Physicians India 49, 377–378 - PubMed
-
- Sorrell T. C., Chen S. C. A., Phillips P., Marr K. A. (2011) Cryptococcus: From Human Pathogen to Model Yeast, pp. 595–606, American Society for Microbiology Press, Washington, D.C
-
- French N., Gray K., Watera C., Nakiyingi J., Lugada E., Moore M., Lalloo D., Whitworth J. A., Gilks C. F. (2002) AIDS 16, 1031–1038 - PubMed
-
- Park B. J., Wannemuehler K. A., Marston B. J., Govender N., Pappas P. G., Chiller T. M. (2009) AIDS 23, 525–530 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

