Stabilization of HIV-1 envelope in the CD4-bound conformation through specific cross-linking of a CD4 mimetic
- PMID: 21487012
- PMCID: PMC3122227
- DOI: 10.1074/jbc.M111.232272
Stabilization of HIV-1 envelope in the CD4-bound conformation through specific cross-linking of a CD4 mimetic
Erratum in
- J Biol Chem. 2011 Aug 19;286(33):29442. Ramos, Oscar H P [added]
Abstract
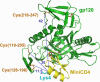
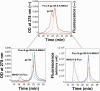
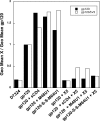
CD4 binding on gp120 leads to the exposure of highly conserved regions recognized by the HIV co-receptor CCR5 and by CD4-induced (CD4i) antibodies. A covalent gp120-CD4 complex was shown to elicit CD4i antibody responses in monkeys, which was correlated with control of the HIV virus infection (DeVico, A., Fouts, T., Lewis, G. K., Gallo, R. C., Godfrey, K., Charurat, M., Harris, I., Galmin, L., and Pal, R. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 17477-17482). Because the inclusion of CD4 in a vaccine formulation should be avoided, due to potential autoimmune reactions, we engineered small sized CD4 mimetics (miniCD4s) that are poorly immunogenic and do not induce anti-CD4 antibodies. We made covalent complexes between such an engineered miniCD4 and gp120 or gp140, through a site-directed coupling reaction. These complexes were recognized by CD4i antibodies as well as by the HIV co-receptor CCR5. In addition, they elicit CD4i antibody responses in rabbits and therefore represent potential vaccine candidates that mimic an important HIV fusion intermediate, without autoimmune hazard.
Figures



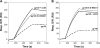

References
-
- Cohen J. (2003) Science 302, 1309–1310 - PubMed
-
- Burton D. R., Desrosiers R. C., Doms R. W., Koff W. C., Kwong P. D., Moore J. P., Nabel G. J., Sodroski J., Wilson I. A., Wyatt R. T. (2004) Nat. Immunol. 5, 233–236 - PubMed
-
- Gallo R. C. (2005) Lancet 366, 1894–1898 - PubMed
-
- Girard M. P., Osmanov S. K., Kieny M. P. (2006) Vaccine 24, 4062–4081 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

