In vivo inhibition of human CD19-targeted effector T cells by natural T regulatory cells in a xenotransplant murine model of B cell malignancy
- PMID: 21487038
- PMCID: PMC3094720
- DOI: 10.1158/0008-5472.CAN-10-0552
In vivo inhibition of human CD19-targeted effector T cells by natural T regulatory cells in a xenotransplant murine model of B cell malignancy
Abstract
Human T cells genetically modified to express chimeric antigen receptors (CAR) specific to the B cell tumor antigen CD19 can successfully eradicate systemic human CD19(+) tumors in immunocompromised SCID (severe combined immunodeficient)-Beige mice. However, in the clinical setting, CD4(+) CD25(hi) T regulatory cells (Treg) present within the tumor microenvironment may be potent suppressors of tumor-targeted effector T cells. In order to assess the impact of Tregs on CAR-modified T cells in the SCID-Beige xenotransplant model, we isolated, genetically targeted and expanded natural T regulatory cells (nTreg). In vitro nTregs modified to express CD19-targeted CARs efficiently inhibited the proliferation of activated human T cells, as well as the capacity of CD19-targeted 19-28z(+) effector T cells to lyse CD19(+) Raji tumor cells. Intravenous infusion of CD19-targeted nTregs into SCID-Beige mice with systemic Raji tumors traffic to sites of tumor and recapitulate a clinically relevant hostile tumor microenvironment. Antitumor efficacy of subsequently infused 19-28z(+) effector T cells was fully abrogated as assessed by long-term survival of treated mice. Optimal suppression by genetically targeted nTregs was dependent on nTreg to effector T-cell ratios and in vivo nTreg activation. Prior infusion of cyclophosphamide in the setting of this nTreg-mediated hostile microenvironment was able to restore the antitumor activity of subsequently infused 19-28z(+) effector T cells through the eradication of tumor-targeted nTregs. These findings have significant implications for the design of future clinical trials utilizing CAR-based adoptive T-cell therapies of cancer.
©2011 AACR.
Figures
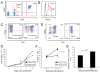


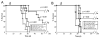

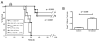
References
-
- Sadelain M, Riviere I, Brentjens R. Targeting tumours with genetically enhanced T lymphocytes. Nat Rev Cancer. 2003;3:35–45. - PubMed
-
- Brentjens RJ, Latouche JB, Santos E, et al. Eradication of systemic B-cell tumors by genetically targeted human T lymphocytes co-stimulated by CD80 and interleukin-15. Nat Med. 2003;9:279–86. - PubMed
-
- Brentjens RJ, Santos E, Nikhamin Y, et al. Genetically targeted T cells eradicate systemic acute lymphoblastic leukemia xenografts. Clin Cancer Res. 2007;13:5426–35. - PubMed
-
- Cheadle EJ, Gilham DE, Hawkins RE. The combination of cyclophosphamide and human T cells genetically engineered to target CD19 can eradicate established B-cell lymphoma. Br J Haematol. 2008;142:65–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P50 CA086438/CA/NCI NIH HHS/United States
- K08 CA095152/CA/NCI NIH HHS/United States
- R25 CA096945/CA/NCI NIH HHS/United States
- R01 CA138738/CA/NCI NIH HHS/United States
- CA95152/CA/NCI NIH HHS/United States
- CA86438/CA/NCI NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- CA096945/CA/NCI NIH HHS/United States
- P01 CA059350/CA/NCI NIH HHS/United States
- CA08748/CA/NCI NIH HHS/United States
- P01 CA094060/CA/NCI NIH HHS/United States
- CA059350/CA/NCI NIH HHS/United States
- CA094060/CA/NCI NIH HHS/United States
- P30 CA008748/CA/NCI NIH HHS/United States
- CA138738/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

