Effectiveness of antenatal corticosteroids in reducing respiratory disorders in late preterm infants: randomised clinical trial
- PMID: 21487057
- PMCID: PMC3075234
- DOI: 10.1136/bmj.d1696
Effectiveness of antenatal corticosteroids in reducing respiratory disorders in late preterm infants: randomised clinical trial
Abstract
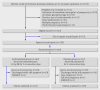
Objectives: To determine the effectiveness of corticosteroids in reducing respiratory disorders in infants born at 34-36 weeks' gestation. Design Randomised triple blind clinical trial. Setting A large tertiary teaching hospital in northeast of Brazil. Participants Women at 34-36 weeks of pregnancy at risk of imminent premature delivery. Interventions Betamethasone 12 mg or placebo intramuscularly for two consecutive days. Main outcomes measures Primary outcome was the incidence of respiratory disorders (respiratory distress syndrome and transient tachypnoea of the newborn). Secondary outcomes included the need for ventilatory support, neonatal morbidity, and duration of stay in hospital.
Results: 320 women were randomised, 163 of whom were assigned to the treatment group and 157 to the controls. Final analysis included 143 and 130 infants, respectively. The rate of respiratory distress syndrome was low (two (1.4%) in the corticosteroid group; one (0.8%) in the placebo group; P = 0.54), while the rate of transient tachypnoea was high in both groups (34 (24%) v 29 (22%); P = 0.77). There was no reduction in the risk of respiratory morbidity with corticosteroid use even after adjustment for subgroups of gestational age (34-34(+6) weeks, 35-35(+6) weeks, and ≥ 36 weeks). The adjusted risk of respiratory morbidity was 1.12 (95% confidence interval 0.74 to 1.70). The need for ventilatory support was around 20% in both groups. There was no difference in neonatal morbidity (88 (62%) v 93 (72%); P = 0.08) or in the duration of stay in hospital between the two groups (5.12 v 5.22 days; P = 0.87). Phototherapy for jaundice was required less often in babies whose mothers received corticosteroids (risk ratio 0.63, 0.44 to 0.91).
Conclusions: Antenatal treatment with corticosteroids at 34-36 weeks of pregnancy does not reduce the incidence of respiratory disorders in newborn infants. Trial registration Clinical Trials NCT00675246.
Conflict of interest statement
Competing interests: All authors have completed the Unified Competing Interest form at
Figures
Comment in
-
Antenatal corticosteroids in late preterm infants.BMJ. 2011 Apr 12;342:d1614. doi: 10.1136/bmj.d1614. BMJ. 2011. PMID: 21487056 No abstract available.
-
The administration of corticosteroids to 34-36-week pregnant women at risk of imminent delivery does not reduce the risk of respiratory disorders in the newborn.Evid Based Med. 2012 Apr;17(2):41-2. doi: 10.1136/ebm.2011.100150. Epub 2011 Sep 26. Evid Based Med. 2012. PMID: 21949260 No abstract available.
References
-
- Young PC, Glasgow TS, Li X, Guest-Warnick G, Stoddard G. Mortality of late-preterm (near-term) newborns in Utah. Pediatrics 2007;119:e659-65. - PubMed
-
- Kramer MS, Demissie K, Yang H, Platt RW, Sauvé R, Liston R. The contribution of mild and moderate preterm birth to infant mortality. Fetal and Infant Health Study Group of the Canadian Perinatal Surveillance System. JAMA 2000;284:843-9. - PubMed
-
- Wang ML, Dorer DJ, Fleming MP, Catlin EA. Clinical outcomes of near-term infants. Pediatrics 2004;114:372-6. - PubMed
-
- Tomashek KM, Shapiro-Mendoza CK, Weiss J, Kotelchuck M, Barfield W, Evans S, et al. Early discharge among late preterm and term newborns and risk of neonatal morbidity. Semin Perinatol 2006;30:61-8. - PubMed
-
- Davidoff MJ, Dias T, Damus K, Russell R, Bettegowda VR, Dolan S, et al. Changes in the gestational age distribution among US singleton births: impact on rates of late preterm birth, 1992 to 2002. Semin Perinatol 2006;30:8-15. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical