Plasma markers for identifying patients with metastatic melanoma
- PMID: 21487066
- PMCID: PMC3415234
- DOI: 10.1158/1078-0432.CCR-10-2402
Plasma markers for identifying patients with metastatic melanoma
Abstract
Purpose: With the rising incidence of melanoma, more patients are undergoing surveillance for disease recurrence. Our purpose was to study levels of proteins that might be secreted in the blood of patients with metastatic melanoma that can be used for monitoring these individuals.
Methods: Genome-wide gene expression data were used to identify abundantly expressed genes in melanoma cells that encode for proteins likely to be present in the blood of cancer patients, based on high expression levels in tumors. ELISA assays were employed to measure proteins in plasma of 216 individuals; 108 metastatic melanoma patients and 108 age- and gender-matched patients with resected stage I/II disease split into equal-sized training and test cohorts.
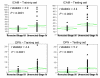
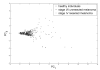
Results: Levels of seven markers, CEACAM (carcinoembryonic antigen-related cell adhesion molecule), ICAM-1 (intercellular adhesion molecule 1), osteopontin, MIA (melanoma inhibitory activity), GDF-15 (growth differentiation factor 15), TIMP-1 (tissue inhibitor of metalloproteinase 1), and S100B, were higher in patients with unresected stage IV disease than in patients with resected stage I/II disease. About 81% of the stage I/II patients in the training set had no marker elevation, whereas 69% of the stage IV patients had elevation of at least one marker (P < 0.0001). Receiver operating characteristic curves for the markers in combination in these two patient populations had an area under curve (AUC) of 0.79 in the training set and 0.8 in the test set. A CART (Classification and Regression Trees) model developed in the training set further improved the AUC in the test set to 0.898.
Conclusions: Plasma markers, particularly when assessed in combination, can be used to monitor patients for disease recurrence and can compliment currently used lactate dehydrogenase and imaging studies; prospective validation is warranted.
©2011 AACR.
Figures


References
-
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49. - PubMed
-
- Ak I, Stokkel MP, Bergman W, Pauwels EK. Cutaneous malignant melanoma: clinical aspects, imaging modalities and treatment. Eur J Nucl Med. 2000;27:447–58. - PubMed
-
- Garbe C, Hauschild A, Volkenandt M, et al. Evidence and interdisciplinary consense-based German guidelines: diagnosis and surveillance of melanoma. Melanoma Res. 2007;17:393–9. - PubMed
-
- Ho Shon IA, Chung DK, Saw RP, Thompson JF. Imaging in cutaneous melanoma. Nucl Med Commun. 2008;29:847–76. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

