Benefit of intensive statin therapy in women: results from PROVE IT-TIMI 22
- PMID: 21487089
- PMCID: PMC3097284
- DOI: 10.1161/CIRCOUTCOMES.110.957720
Benefit of intensive statin therapy in women: results from PROVE IT-TIMI 22
Abstract
Background: Despite the known benefit of intensive statin therapy for reducing future cardiovascular events, its effectiveness in women has been questioned by some.
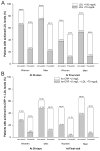
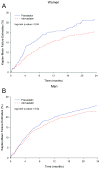
Methods and results: In the Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 (PROVE IT-TIMI 22) trial, 911 (21.9%) women and 3251 (78.1%) men were randomized to intensive statin (atorvastatin 80 mg) versus standard therapy (pravastatin 40 mg) therapy for a median duration of 2.1 years. The primary end point was death, myocardial infarction, unstable angina; revascularization (occurring after 30 days); or stroke. Safety end points included elevations in liver function tests, creatine kinase, and myalgias/myositis. Women had a reduction in low-density lipoprotein (LDL) of 42.8% from baseline at 30 days (to a median of 60 mg/dL) in the intensive therapy arm, with 88.8% reaching the LDL goal of <100 mg/dL and 65.0% of <70 mg/dL, compared with a 16.8% reduction in LDL (to a median of 88 mg/dL) in the standard therapy arm. Women receiving intensive statin therapy had a significant 25% relative reduction over standard dose (hazard ratio, 0.75; 95% CI, 0.57 to 0.99; P=0.04) for the primary composite end point compared with a 14% reduction for men (hazard ratio, 0.86; 95% CI, 0.75 to 0.99; P=0.04; P-interaction, 0.38). No differences were observed between sexes for safety (all P-interaction ≥0.11).
Conclusions: This trial provides evidence that both women and men derived benefit from intensive statin therapy after acute coronary syndrome, and thus, sex should not be a factor in determining who should be treated with intensive statin therapy.
Trial registration: ClinicalTrials.gov NCT00382460.
Conflict of interest statement
Figures


Comment in
-
Clinical trial subgroups: challenges and opportunities in describing the benefits of therapy.Circ Cardiovasc Qual Outcomes. 2011 May;4(3):266-7. doi: 10.1161/CIRCOUTCOMES.111.961128. Circ Cardiovasc Qual Outcomes. 2011. PMID: 21586724 No abstract available.
References
-
- Mosca L, Banka CL, Benjamin EJ, Berra K, Bushnell C, Dolor RJ, Ganiats TG, Gomes AS, Gornik HL, Gracia C, Gulati M, Haan CK, Judelson DR, Keenan N, Kelepouris E, Michos ED, Newby LK, Oparil S, Ouyang P, Oz MC, Petitti D, Pinn VW, Redberg RF, Scott R, Sherif K, Smith SC, Jr, Sopko G, Steinhorn RH, Stone NJ, Taubert KA, Todd BA, Urbina E, Wenger NK. Evidence-based guidelines for cardiovascular disease prevention in women: 2007 update. J Am Coll Cardiol. 2007;49:1230–1250. - PubMed
-
- Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger V, Rosamond W, Sacco R, Sorlie P, Stafford R, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J. Heart Disease and Stroke Statistics--2010 Update. A Report From the American Heart Association. Circulation. 2009 - PubMed
-
- Downs JR, Clearfield M, Weis S, Whitney E, Shapiro DR, Beere PA, Langendorfer A, Stein EA, Kruyer W, Gotto AM., Jr Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study JAMA. 1998;279:1615–1622. - PubMed
-
- Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S) Lancet. 1994;344:1383–1389. - PubMed
-
- Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. N Engl J Med. 1998;339:1349–1357. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

