Fragmentation of monoclonal antibodies
- PMID: 21487244
- PMCID: PMC3149706
- DOI: 10.4161/mabs.3.3.15608
Fragmentation of monoclonal antibodies
Abstract
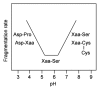
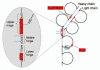
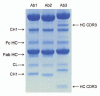
Fragmentation is a degradation pathway ubiquitously observed in proteins despite the remarkable stability of peptide bond; proteins differ only by how much and where cleavage occurs. The goal of this review is to summarize reports regarding the non-enzymatic fragmentation of the peptide backbone of monoclonal antibodies (mAbs). The sites in the polypeptide chain susceptible to fragmentation are determined by a multitude of factors. Insights are provided on the intimate chemical mechanisms that can make some bonds prone to cleavage due to the presence of specific side-chains. In addition to primary structure, the secondary, tertiary and quaternary structures have a significant impact in modulating the distribution of cleavage sites by altering local flexibility, accessibility to solvent or bringing in close proximity side chains that are remote in sequence. This review focuses on cleavage sites observed in the constant regions of mAbs, with special emphasis on hinge fragmentation. The mechanisms responsible for backbone cleavage are strongly dependent on pH and can be catalyzed by metals or radicals. The distribution of cleavage sites are different under acidic compared to basic conditions, with fragmentation rates exhibiting a minimum in the pH range 5 to 6; therefore, the overall fragmentation pattern observed for a mAb is a complex result of structural and solvent conditions. A critical review of the techniques used to monitor fragmentation is also presented; usually a compromise has to be made between a highly sensitive method with good fragment separation and the capability to identify the cleavage site. The effect of fragmentation on the function of a mAb must be evaluated on a case-by-case basis depending on whether cleavage sites are observed in the variable or constant regions, and on the mechanism of action of the molecule.
Figures



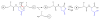
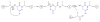

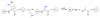

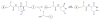
References
-
- Cordoba AJ, Shyong BJ, Breen D, Harris RJ. Nonenzymatic hinge region fragmentation of antibodies in solution. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;818:115–121. - PubMed
-
- Kamerzell TJ, Li M, Arora S, Ji JA, Wang YJ. The relative rate of immunoglobulin gamma 1 fragmentation. J Pharm Sci. 2010;100:1341–1349. - PubMed
-
- Bernard Testa, Mayer JM. Hydrolysis in Drug and Prodrug Metabolism. Verlag Helvetica Chimica Acta and Wiley-VCH; 2003.
-
- Liu H, Gaza-Bulseco G, Lundell E. Assessment of antibody fragmentation by reversed-phase liquid chromatography and mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;876:13–23. - PubMed
-
- Dillon TM, Bondarenko PV, Rehder DS, Pipes GD, Kleemann GR, Ricci MS. Optimization of a reversedphase high-performance liquid chromatography/mass spectrometry method for characterizing recombinant antibody heterogeneity and stability. J Chromatogr A. 2006;1120:112–120. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
