Efficacy of NNRTI-based antiretroviral therapy initiated during acute HIV infection
- PMID: 21487250
- PMCID: PMC3569481
- DOI: 10.1097/QAD.0b013e3283463c07
Efficacy of NNRTI-based antiretroviral therapy initiated during acute HIV infection
Abstract
Objective: Characterize responses to non-nucleoside reverse transcriptase inhibitor (NNRTI)-based antiretroviral treatment (ART) initiated during acute HIV infection (AHI).
Design: This was a prospective, single-arm evaluation of once-daily, co-formulated emtricitabine/tenofovir/efavirenz initiated during AHI.
Methods: The primary endpoint is the proportion of responders with HIV RNA less than 200 copies/ml by week 24. We examined time to viral suppression and CD8 cell activation in relation to baseline participant characteristics. We compared time to viral suppression and viral dynamics using linear mixed-effects models between acutely infected participants and chronically infected controls.
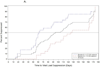
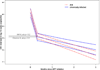
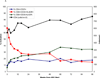
Results: Between January 2005 and May 2009, 61 AHI participants were enrolled. Of participants whose enrollment date allowed 24 and 48 weeks of follow-up, 47 of 51 (92%) achieved viral suppression to less than 200 copies/ml by week 24, and 35 of 41 (85.4%) to less than 50 copies/ml by week 48. The median time from ART initiation to suppression below 50 copies/ml was 93 days (range 14-337). Higher HIV RNA levels at ART initiation (P = 0.02), but not time from estimated date of infection to ART initiation (P = 0.86), were associated with longer time to viral suppression. The median baseline frequency of activated CD8+CD38+HLA-DR+ T cells was 67% (range 40-95), and was not significantly associated with longer time to viral load suppression (P = 0.15). Viremia declined to less than 50 copies/ml more rapidly in AHI than chronically infected participants. Mixed-model analysis demonstrated similar phase I HIV RNA decay rates between acute and chronically infected participants, and more rapid viral decline in acutely infected participants in phase II.
Conclusion: Once-daily emtricitabine/tenofovir/efavirenz initiated during AHI achieves rapid and sustained HIV suppression during this highly infectious period.
Conflict of interest statement
Figures


References
-
- Powers K, Ghani A, Miller W, Hoffman I, Pettifor A, Kamanga G, et al. The contribution of early HIV infection to HIV spread in Lilongwe, Malawi: implications for transmission prevention strategies. XVIII International AIDS Conference; Vienna, Austria. 2010.
-
- Chakraborty H, Sen PK, Helms RW, Vernazza PL, Fiscus SA, Eron JJ, et al. Viral burden in genital secretions determines male-to-female sexual transmission of HIV-1: a probabilistic empiric model. AIDS. 2001;15:621–627. - PubMed
-
- Quinn TC, Wawer MJ, Sewankambo N, Serwadda D, Li C, Wabwire-Mangen F, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med. 2000;342:921–929. - PubMed
-
- Tovanabutra S, Robison V, Wongtrakul J, Sennum S, Suriyanon V, Kingkeow D, et al. Male viral load and heterosexual transmission of HIV-1 subtype E in northern Thailand. J Acquir Immune Defic Syndr. 2002;29:275–283. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

