Drug-induced caspase 8 upregulation sensitises cisplatin-resistant ovarian carcinoma cells to rhTRAIL-induced apoptosis
- PMID: 21487429
- PMCID: PMC3078595
- DOI: 10.1038/bjc.2011.84
Drug-induced caspase 8 upregulation sensitises cisplatin-resistant ovarian carcinoma cells to rhTRAIL-induced apoptosis
Abstract
Background: Drug resistance is a major problem in ovarian cancer. Triggering apoptosis using death ligands such as tumour necrosis factor-related apoptosis inducing ligand (TRAIL) might overcome chemoresistance.
Methods: We investigated whether acquired cisplatin resistance affects sensitivity to recombinant human (rh) TRAIL alone or in combination with cisplatin in an ovarian cancer cell line model consisting of A2780 and its cisplatin-resistant subline CP70.
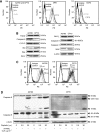
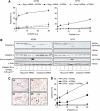
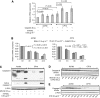
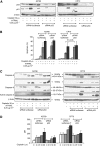
Results: Combining cisplatin and rhTRAIL strongly enhanced apoptosis in both cell lines. CP70 expressed less caspase 8 protein, whereas mRNA levels were similar compared with A2780. Pre-exposure of particularly CP70 to cisplatin resulted in strongly elevated caspase 8 protein and mRNA levels. Caspase 8 mRNA turnover and protein stability in the presence or absence of cisplatin did not differ between both cell lines. Cisplatin-induced caspase 8 protein levels were essential for the rhTRAIL-sensitising effect as demonstrated using caspase 8 small-interfering RNA (siRNA) and caspase-8 overexpressing constructs. Cellular FLICE-inhibitory protein (c-FLIP) and p53 siRNA experiments showed that neither an altered caspase 8/c-FLIP ratio nor a p53-dependent increase in DR5 membrane expression following cisplatin were involved in rhTRAIL sensitisation.
Conclusion: Cisplatin enhances rhTRAIL-induced apoptosis in cisplatin-resistant ovarian cancer cells, and induction of caspase 8 protein expression is the key factor of rhTRAIL sensitisation.
Figures


References
-
- Abedini MR, Muller EJ, Brun J, Bergeron R, Gray DA, Tsang BK (2008) Cisplatin induces p53-dependent FLICE-like inhibitory protein ubiquitination in ovarian cancer cells. Cancer Res 68: 4511–4517 - PubMed
-
- Abedini MR, Qiu Q, Yan X, Tsang BK (2004) Possible role of FLICE-like inhibitory protein (FLIP) in chemoresistant ovarian cancer cells in vitro. Oncogene 23: 6997–7004 - PubMed
-
- Agarwal R, Kaye SB (2003) Ovarian cancer: strategies for overcoming resistance to chemotherapy. Nat Rev Cancer 3: 502–516 - PubMed
-
- Ashkenazi A, Holland P, Eckhardt SG (2008) Ligand-based targeting of apoptosis in cancer: the potential of recombinant human apoptosis ligand 2/tumor necrosis factor-related apoptosis-inducing ligand (rhApo2L/TRAIL). J Clin Oncol 26: 3621–3630 - PubMed
-
- Ashkenazi A, Pai RC, Fong S, Leung S, Lawrence DA, Marsters SA, Blackie C, Chang L, McMurtrey AE, Hebert A, DeForge L, Koumenis IL, Lewis D, Harris L, Bussiere J, Koeppen H, Shahrokh Z, Schwall RH (1999) Safety and antitumor activity of recombinant soluble Apo2 ligand. J Clin Invest 104: 155–162 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

