Pyrene-functionalized oligonucleotides and locked nucleic acids (LNAs): tools for fundamental research, diagnostics, and nanotechnology
- PMID: 21487621
- PMCID: PMC3644995
- DOI: 10.1039/c1cs15014f
Pyrene-functionalized oligonucleotides and locked nucleic acids (LNAs): tools for fundamental research, diagnostics, and nanotechnology
Abstract
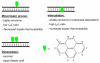
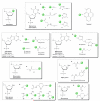
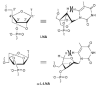
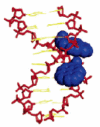
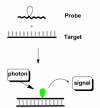
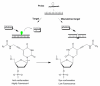
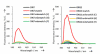
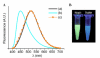
Pyrene-functionalized oligonucleotides (PFOs) are increasingly explored as tools in fundamental research, diagnostics and nanotechnology. Their popularity is linked to the ability of pyrenes to function as polarity-sensitive and quenchable fluorophores, excimer-generating units, aromatic stacking moieties and nucleic acid duplex intercalators. These characteristics have enabled development of PFOs for detection of complementary DNA/RNA targets, discrimination of single nucleotide polymorphisms (SNPs), and generation of π-arrays on nucleic acid scaffolds. This critical review will highlight the physical properties and applications of PFOs that are likely to provide high degree of positional control of the chromophore in nucleic acid complexes. Particular emphasis will be placed on pyrene-functionalized Locked Nucleic Acids (LNAs) since these materials display interesting properties such as fluorescence quantum yields approaching unity and recognition of mixed-sequence double stranded DNA (144 references).
Figures

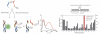

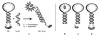

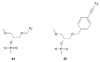

Similar articles
-
Recent Advances in Nucleic Acid Targeting Probes and Supramolecular Constructs Based on Pyrene-Modified Oligonucleotides.Molecules. 2017 Nov 30;22(12):2108. doi: 10.3390/molecules22122108. Molecules. 2017. PMID: 29189716 Free PMC article. Review.
-
Scaffolding along nucleic acid duplexes using 2'-amino-locked nucleic acids.Acc Chem Res. 2014 Jun 17;47(6):1768-77. doi: 10.1021/ar500014g. Epub 2014 Apr 21. Acc Chem Res. 2014. PMID: 24749544
-
Highly fluorescent conjugated pyrenes in nucleic acid probes: (phenylethynyl)pyrenecarbonyl-functionalized locked nucleic acids.Chemistry. 2008;14(35):11010-26. doi: 10.1002/chem.200801077. Chemistry. 2008. PMID: 18979465
-
Identification and characterization of second-generation invader locked nucleic acids (LNAs) for mixed-sequence recognition of double-stranded DNA.J Org Chem. 2013 Oct 4;78(19):9560-70. doi: 10.1021/jo4015936. Epub 2013 Sep 25. J Org Chem. 2013. PMID: 24032477 Free PMC article.
-
25 years and still going strong: 2'-O-(pyren-1-yl)methylribonucleotides - versatile building blocks for applications in molecular biology, diagnostics and materials science.Org Biomol Chem. 2017 Nov 29;15(46):9760-9774. doi: 10.1039/c7ob02152f. Org Biomol Chem. 2017. PMID: 29135014 Free PMC article. Review.
Cited by
-
Fluorescent Probes for Selective Recognition of Hypobromous Acid: Achievements and Future Perspectives.Molecules. 2021 Jan 12;26(2):363. doi: 10.3390/molecules26020363. Molecules. 2021. PMID: 33445736 Free PMC article. Review.
-
Conformational Control of Dual Emission by Pyrrolidinyl PNA-DNA Hybrids.ChemistryOpen. 2012 Aug;1(4):173-6. doi: 10.1002/open.201200016. Epub 2012 Jul 11. ChemistryOpen. 2012. PMID: 24551507 Free PMC article. No abstract available.
-
DNA Strand Displacement with Base Pair Stabilizers: Purine-2,6-Diamine and 8-Aza-7-Bromo-7-Deazapurine-2,6-Diamine Oligonucleotides Invade Canonical DNA and New Fluorescent Pyrene Click Sensors Monitor the Reaction.Chemistry. 2022 Dec 27;28(72):e202202412. doi: 10.1002/chem.202202412. Epub 2022 Nov 15. Chemistry. 2022. PMID: 36178316 Free PMC article.
-
Novel Tripodal Polyamine Tris-Pyrene: DNA/RNA Binding and Photodynamic Antiproliferative Activity.Pharmaceutics. 2023 Aug 25;15(9):2197. doi: 10.3390/pharmaceutics15092197. Pharmaceutics. 2023. PMID: 37765167 Free PMC article.
-
Influence of perylenediimide-pyrene supramolecular interactions on the stability of DNA-based hybrids: Importance of electrostatic complementarity.Beilstein J Org Chem. 2014 Jul 11;10:1589-95. doi: 10.3762/bjoc.10.164. eCollection 2014. Beilstein J Org Chem. 2014. PMID: 25161715 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

