Serum and liver iron differently regulate the bone morphogenetic protein 6 (BMP6)-SMAD signaling pathway in mice
- PMID: 21488083
- PMCID: PMC3277401
- DOI: 10.1002/hep.24359
Serum and liver iron differently regulate the bone morphogenetic protein 6 (BMP6)-SMAD signaling pathway in mice
Abstract
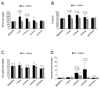
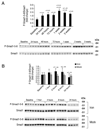
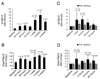
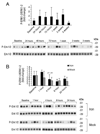
The bone morphogenetic protein 6 (BMP6)-SMAD signaling pathway is a central regulator of hepcidin expression and systemic iron balance. However, the molecular mechanisms by which iron is sensed to regulate BMP6-SMAD signaling and hepcidin expression are unknown. Here we examined the effects of circulating and tissue iron on Bmp6-Smad pathway activation and hepcidin expression in vivo after acute and chronic enteral iron administration in mice. We demonstrated that both transferrin saturation and liver iron content independently influence hepcidin expression. Although liver iron content is independently positively correlated with hepatic Bmp6 messenger RNA (mRNA) expression and overall activation of the Smad1/5/8 signaling pathway, transferrin saturation activates the downstream Smad1/5/8 signaling cascade, but does not induce Bmp6 mRNA expression in the liver. Hepatic inhibitory Smad7 mRNA expression is increased by both acute and chronic iron administration and mirrors overall activation of the Smad1/5/8 signaling cascade. In contrast to the Smad pathway, the extracellular signal-regulated kinase 1 and 2 (Erk1/2) mitogen-activated protein kinase (Mapk) signaling pathway in the liver is not activated by acute or chronic iron administration in mice.
Conclusion: Our data demonstrate that the hepatic Bmp6-Smad signaling pathway is differentially activated by circulating and tissue iron to induce hepcidin expression, whereas the hepatic Erk1/2 signaling pathway is not activated by iron in vivo.
Copyright © 2011 American Association for the Study of Liver Diseases.
Figures



Comment in
-
Liver and serum iron: discrete regulators of hepatic hepcidin expression.Hepatology. 2011 Jul;54(1):16-9. doi: 10.1002/hep.24449. Hepatology. 2011. PMID: 21618572 No abstract available.
References
-
- Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090–2093. - PubMed
-
- Pigeon C, Ilyin G, Courselaud B, Leroyer P, Turlin B, Brissot P, et al. A new mouse liver-specific gene, encoding a protein homologous to human antimicrobial peptide hepcidin, is overexpressed during iron overload. J Biol Chem. 2001;276:7811–7819. - PubMed
-
- Nemeth E, Valore EV, Territo M, Schiller G, Lichtenstein A, Ganz T. Hepcidin, a putative mediator of anemia of inflammation, is a type II acute-phase protein. Blood. 2003;101:2461–2463. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
