Spatial and temporal changes in promoter activity of the astrocyte glutamate transporter GLT1 following traumatic spinal cord injury
- PMID: 21488085
- PMCID: PMC9162082
- DOI: 10.1002/jnr.22624
Spatial and temporal changes in promoter activity of the astrocyte glutamate transporter GLT1 following traumatic spinal cord injury
Abstract
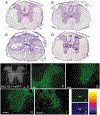
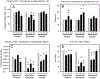
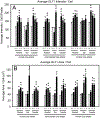
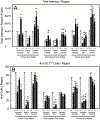
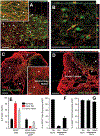
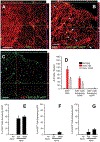
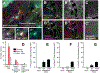
After traumatic spinal cord injury (SCI), there is an opportunity for preserving function by attenuating secondary cell loss. Astrocytes play crucial roles in the adult CNS and are responsible for the vast majority of glutamate buffering, potentially preventing excitotoxic loss of neurons and oligodendrocytes. We examined spatial and temporal changes in gene expression of the major astrocyte glutamate transporter GLT1 following moderate thoracic contusion SCI using transgenic BAC-GLT1-eGFP promoter reporter mice. In dorsal column white matter, total intensity of GLT1-eGFP expression per region was significantly reduced following SCI at both lesion epicenter and at rostral and caudal areas where no tissue loss occurred. This regional decrease in GLT1 expression was due to significant loss of GLT1-eGFP(+) cells, partially accounted for by apoptosis of eGFP(+) /GFAP(+) astrocytes in both white and gray matter. There were also decreased numbers of GLT1-eGFP-expressing cells in multiple gray matter regions following injury; nevertheless, there was sustained or even increased regional GLT1-eGFP expression in gray matter as a result of up-regulation in astrocytes that continued to express GLT1-eGFP. Although there were increased numbers of GFAP(+) cells both at the lesion site and in surrounding intact spinal cord following SCI, the majority of proliferating Ki67(+) /GFAP(+) astrocytes did not express GLT1-eGFP. These findings demonstrate that spatial and temporal alterations in GLT1 expression observed after SCI result from both astrocyte death and gene expression changes in surviving astrocytes. Results also suggest that following SCI a significant portion of astrocytes lacks GLT1 expression, possibly compromising the important role of astrocytes in glutamate homeostasis.
Copyright © 2011 Wiley-Liss, Inc.
Figures

References
-
- Baptiste DC, Fehlings MG. 2006. Pharmacological approaches to repair the injured spinal cord. J Neurotrauma 23:318–334. - PubMed
-
- Basso DM, Fisher LC, Anderson AJ, Jakeman LB, McTigue DM, Popovich PG. 2006. Basso Mouse Scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J Neurotrauma 23:635–659. - PubMed
-
- Cao H, Zhang YQ. 2008. Spinal glial activation contributes to pathological pain states. Neurosci Biobehav Rev 32:972–983. - PubMed
-
- Danbolt NC. 2001. Glutamate uptake. Prog Neurobiol 65:1–105. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

