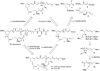
Total synthesis of iejimalide B
- PMID: 21488673
- PMCID: PMC3123399
- DOI: 10.1021/jo200514m
Total synthesis of iejimalide B
Abstract
Iejimalide B, a structurally unique 24-membered polyene macrolide having a previously underutilized mode of anticancer activity, was synthesized according to a strategy employing Julia-Kocienski olefinations, a palladium-catalyzed Heck reaction, a palladium-catalyzed Marshall propargylation, a Keck-type esterification, and a palladium-catalyzed macrolide-forming, intramolecular Stille coupling of a highly complex cyclization substrate. The overall synthesis is efficient (19.5% overall yield for 15 linear steps) and allows for more practical scaled-up synthesis than previously reported strategies that differed in the order of assembly of key subunits and in the method of macrocyclization. The present synthesis paves the way for efficient preparation of analogues for drug development efforts.
Figures
References
-
- Kobayashi J, Cheng J, Ohta T, Nakamura H, Nozoe S, Hirata Y, Ohizumi Y, Sasaki T. J. Org. Chem. 1988;53:6147–6150.
- Kikuchi Y, Ishibashi M, Sasaki T, Kobayashi J. Tetrahedron Lett. 1991;32 797-796.
- Tsuda M, Nozawa K, Shimbo K, Ishiyama H, Fukushi E, Kawabata J, Kobayashi J. Tetrahedron Lett. 2003;44:1395–1399.
- Nozawa K, Tsuda M, Ishiyama H, Sasaki T, Tsuruo T, Kobayashi J. Bioorg. Med. Chem. 2006;14:1063–1067. - PubMed
-
- Forgac M. Nat. Rev. Mol. Cell Biol. 2007;8:917–929. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources