Acute serum hormone levels: characterization and prognosis after severe traumatic brain injury
- PMID: 21488721
- PMCID: PMC3113446
- DOI: 10.1089/neu.2010.1586
Acute serum hormone levels: characterization and prognosis after severe traumatic brain injury
Abstract
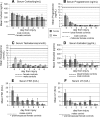
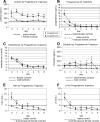
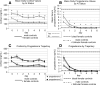
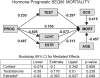
Experimental traumatic brain injury (TBI) studies report the neuroprotective effects of female sex steroids on multiple mechanisms of injury, with the clinical assumption that women have hormonally mediated neuroprotection because of the endogenous presence of these hormones. Other literature indicates that testosterone may exacerbate injury. Further, stress hormone abnormalities that accompany critical illness may both amplify or blunt sex steroid levels. To better understand the role of sex steroid exposure in mediating TBI, we 1) characterized temporal profiles of serum gonadal and stress hormones in a population with severe TBI during the acute phases of their injury; and 2) used a biological systems approach to evaluate these hormones as biomarkers predicting global outcome. The study population was 117 adults (28 women; 89 men) with severe TBI. Serum samples (n=536) were collected for 7 days post-TBI for cortisol, progesterone, testosterone, estradiol, luteinizing hormone (LH), and follicle-stimulating hormone (FSH). Hormone data were linked with clinical data, including acute care mortality and Glasgow Outcome Scale (GOS) scores at 6 months. Hormone levels after TBI were compared to those in healthy controls (n=14). Group based trajectory analysis (TRAJ) was used to develop temporal hormone profiles that delineate distinct subpopulations in the cohort. Structural equations models were used to determine inter-relationships between hormones and outcomes within a multivariate model. Compared to controls, acute serum hormone levels were significantly altered after severe TBI. Changes in the post-TBI adrenal response and peripheral aromatization influenced hormone TRAJ profiles and contributed to the abnormalities, including increased estradiol in men and increased testosterone in women. In addition to older age and greater injury severity, increased estradiol and testosterone levels over time were associated with increased mortality and worse global outcome for both men and women. These findings represent a paradigm shift when thinking about the role of sex steroids in neuroprotection clinically after TBI.
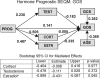
Figures





References
-
- Agha A. Rogers B. Sherlock M. O'Kelly P. Tormey W. Phillips J. Thompson C.J. Anterior pituitary dysfunction in survivors of traumatic brain injury. J. Clin. Endocrinol. Metab. 2004;89:4929–4936. - PubMed
-
- Azcoitia I. Sierra A. Veiga S. Garcia–Segura L.M. Aromatase expression by reactive astroglia is neuroprotective. Ann. N. Y. Acad. Sci. 2003;1007:298–305. - PubMed
-
- Bavisetty S. McArthur D.L. Dusick J.R. Wang C. Cohan P. Boscardin W.J. Swerdloff R. Levin H. Chang D.J. Muizelaar J.P. Kelly D.F. Chronic hypopituitarism after traumatic brain injury: risk assessment and relationship to outcome. Neurosurgery. 2008;62:1080–1093. - PubMed
-
- Beale E. Zhu J. Belzberg H. Changes in serum cortisol with age in critically ill patients. Gerontology. 2002;48:84–92. - PubMed
-
- Behan L.A. Phillips J. Thompson C.J. Agha A. Neuroendocrine disorders after traumatic brain injury. J. Neurol. Neurosurg. Psychiatr. 2008;79:753–759. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

