HIV-1 accessory protein Vpr: relevance in the pathogenesis of HIV and potential for therapeutic intervention
- PMID: 21489275
- PMCID: PMC3090340
- DOI: 10.1186/1742-4690-8-25
HIV-1 accessory protein Vpr: relevance in the pathogenesis of HIV and potential for therapeutic intervention
Abstract
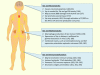
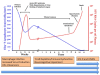
The HIV protein, Vpr, is a multifunctional accessory protein critical for efficient viral infection of target CD4+ T cells and macrophages. Vpr is incorporated into virions and functions to transport the preintegration complex into the nucleus where the process of viral integration into the host genome is completed. This action is particularly important in macrophages, which as a result of their terminal differentiation and non-proliferative status, would be otherwise more refractory to HIV infection. Vpr has several other critical functions including activation of HIV-1 LTR transcription, cell-cycle arrest due to DCAF-1 binding, and both direct and indirect contributions to T-cell dysfunction. The interactions of Vpr with molecular pathways in the context of macrophages, on the other hand, support accumulation of a persistent reservoir of HIV infection in cells of the myeloid lineage. The role of Vpr in the virus life cycle, as well as its effects on immune cells, appears to play an important role in the immune pathogenesis of AIDS and the development of HIV induced end-organ disease. In view of the pivotal functions of Vpr in virus infection, replication, and persistence of infection, this protein represents an attractive target for therapeutic intervention.
Figures

Similar articles
-
Illuminating the Role of Vpr in HIV Infection of Myeloid Cells.Front Immunol. 2019 Jul 23;10:1606. doi: 10.3389/fimmu.2019.01606. eCollection 2019. Front Immunol. 2019. PMID: 31396206 Free PMC article. Review.
-
HIV-1 accessory proteins: VpR.Methods Mol Biol. 2014;1087:125-34. doi: 10.1007/978-1-62703-670-2_11. Methods Mol Biol. 2014. PMID: 24158819 Free PMC article.
-
Quantitative Temporal Viromics of an Inducible HIV-1 Model Yields Insight to Global Host Targets and Phospho-Dynamics Associated with Protein Vpr.Mol Cell Proteomics. 2017 Aug;16(8):1447-1461. doi: 10.1074/mcp.M116.066019. Epub 2017 Jun 12. Mol Cell Proteomics. 2017. PMID: 28606917 Free PMC article.
-
Virion-Associated Vpr Alleviates a Postintegration Block to HIV-1 Infection of Dendritic Cells.J Virol. 2017 Jun 9;91(13):e00051-17. doi: 10.1128/JVI.00051-17. Print 2017 Jul 1. J Virol. 2017. PMID: 28424288 Free PMC article.
-
HIV1-viral protein R (Vpr) mutations: associated phenotypes and relevance for clinical pathologies.Rev Med Virol. 2016 Sep;26(5):314-29. doi: 10.1002/rmv.1889. Epub 2016 Jun 6. Rev Med Virol. 2016. PMID: 27264019 Review.
Cited by
-
Grande retrotransposons contain an accessory gene in the unusually long 3'-internal region that encodes a nuclear protein transcribed from its own promoter.Plant Mol Biol. 2013 Apr;81(6):541-51. doi: 10.1007/s11103-013-0019-2. Epub 2013 Feb 20. Plant Mol Biol. 2013. PMID: 23423698
-
The human antiviral factor TRIM11 is under the regulation of HIV-1 Vpr.PLoS One. 2014 Aug 8;9(8):e104269. doi: 10.1371/journal.pone.0104269. eCollection 2014. PLoS One. 2014. PMID: 25105968 Free PMC article.
-
Clearly different mechanisms of enhancement of short-lived Nef-mediated viral infectivity between SIV and HIV-1.Virol J. 2014 Dec 18;11:222. doi: 10.1186/s12985-014-0222-z. Virol J. 2014. PMID: 25519983 Free PMC article.
-
Deployment of the human immunodeficiency virus type 1 protein arsenal: combating the host to enhance viral transcription and providing targets for therapeutic development.J Gen Virol. 2012 Jun;93(Pt 6):1151-1172. doi: 10.1099/vir.0.041186-0. Epub 2012 Mar 14. J Gen Virol. 2012. PMID: 22422068 Free PMC article.
-
14-3-3s are potential biomarkers for HIV-related neurodegeneration.J Neurovirol. 2012 Oct;18(5):341-53. doi: 10.1007/s13365-012-0121-2. Epub 2012 Jul 19. J Neurovirol. 2012. PMID: 22811265 Free PMC article. Review.
References
-
- Cohen EA, Terwilliger EF, Jalinoos Y, Proulx J, Sodroski JG, Haseltine WA. Identification of HIV-1 vpr product and function. J Acquir Immune Defic Syndr. 1990;3:11–18. - PubMed
-
- Yuan X, Matsuda Z, Matsuda M, Essex M, Lee TH. Human immunodeficiency virus vpr gene encodes a virion-associated protein. AIDS Res Hum Retroviruses. 1990;6:1265–1271. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

