Tocotrienols-induced inhibition of platelet thrombus formation and platelet aggregation in stenosed canine coronary arteries
- PMID: 21489303
- PMCID: PMC3096575
- DOI: 10.1186/1476-511X-10-58
Tocotrienols-induced inhibition of platelet thrombus formation and platelet aggregation in stenosed canine coronary arteries
Abstract
Background: Dietary supplementation with tocotrienols has been shown to decrease the risk of coronary artery disease. Tocotrienols are plant-derived forms of vitamin E, which have potent anti-inflammatory, antioxidant, anticancer, hypocholesterolemic, and neuroprotective properties. Our objective in this study was to determine the extent to which tocotrienols inhibit platelet aggregation and reduce coronary thrombosis, a major risk factor for stroke in humans. The present study was carried out to determine the comparative effects of α-tocopherol, α-tocotrienol, or tocotrienol rich fraction (TRF; a mixture of α-+γ-+δ-tocotrienols) on in vivo platelet thrombosis and ex vivo platelet aggregation (PA) after intravenous injection in anesthetized dogs, by using a mechanically stenosed circumflex coronary artery model (Folts' cyclic flow model).
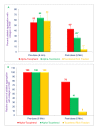
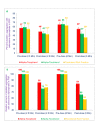
Results: Collagen-induced platelet aggregation (PA) in platelet rich plasma (PRP) was decreased markedly after treatment with α-tocotrienol (59%; P<0.001) and TRF (92%; P<0.001). α-Tocopherol treatment was less effective, producing only a 22% (P<0.05) decrease in PA. Adenosine diphosphate-induced (ADP) PA was also decreased after treatment with α-tocotrienol (34%; P<0.05) and TRF (42%; P<0.025). These results also indicate that intravenously administered tocotrienols were significantly better than tocopherols in inhibiting cyclic flow reductions (CFRs), a measure of the acute platelet-mediated thrombus formation. Tocotrienols (TRF) given intravenously (10 mg/kg), abolished CFRs after a mean of 68 min (range 22 -130 min), and this abolition of CFRs was sustained throughout the monitoring period (50-160 min).Next, pharmacokinetic studies were carried out and tocol levels in canine plasma and platelets were measured. As expected, α-Tocopherol treatment increased levels of total tocopherols in post- vs pre-treatment specimens (57 vs 18 μg/mL in plasma, and 42 vs 10 μg/mL in platelets). However, treatment with α-tocopherol resulted in slightly decreased levels of tocotrienols in post- vs pre-treatment samples (1.4 vs 2.9 μg/mL in plasma and 2.3 vs 2.8 μg/mL in platelets). α-Tocotrienol treatment increased levels of both tocopherols and tocotrienols in post- vs pre-treatment samples (tocopherols, 45 vs 10 μg/mL in plasma and 28 vs 5 μg/mL in platelets; tocotrienols, 2.8 vs 0.9 μg/mL in plasma and 1.28 vs 1.02 μg/mL in platelets). Treatment with tocotrienols (TRF) also increased levels of tocopherols and tocotrienols in post- vs pre-treatment samples (tocopherols, 68 vs 20 μg/mL in plasma and 31.4 vs 7.9 μg/mL in platelets; tocotrienols, 8.6 vs 1.7 μg/mL in plasma and 3.8 vs 3.9 μg/mL in platelets).
Conclusions: The present results indicate that intravenously administered tocotrienols inhibited acute platelet-mediated thrombus formation, and collagen and ADP-induced platelet aggregation. α-Tocotrienols treatment induced increases in α-tocopherol levels of 4-fold and 6-fold in plasma and platelets, respectively. Interestingly, tocotrienols (TRF) treatment induced a less pronounced increase in the levels of tocotrienols in plasma and platelets, suggesting that intravenously administered tocotrienols may be converted to tocopherols. Tocotrienols, given intravenously, could potentially prevent pathological platelet thrombus formation and thus provide a therapeutic benefit in conditions such as stroke and myocardial infarction.
Figures

Similar articles
-
High-dose droperidol protects against experimental coronary thrombosis in dogs and pigs and attenuates aggregation of porcine platelets and Ca2+ mobilization in human platelets.Anesthesiology. 1993 Apr;78(4):733-43. doi: 10.1097/00000542-199304000-00017. Anesthesiology. 1993. PMID: 8466073
-
Antithrombotic effects of MK-0852, a platelet fibrinogen receptor antagonist, in canine models of thrombosis.J Pharmacol Exp Ther. 1993 Sep;266(3):1501-11. J Pharmacol Exp Ther. 1993. PMID: 8371153
-
Mixed tocopherols inhibit platelet aggregation in humans: potential mechanisms.Am J Clin Nutr. 2003 Mar;77(3):700-6. doi: 10.1093/ajcn/77.3.700. Am J Clin Nutr. 2003. PMID: 12600864 Clinical Trial.
-
An in vivo model of experimental arterial stenosis, intimal damage, and periodic thrombosis.Circulation. 1991 Jun;83(6 Suppl):IV3-14. Circulation. 1991. PMID: 2040070 Review.
-
Influence of vitamin E on platelet function in humans.J Am Coll Nutr. 1991 Oct;10(5):466-73. doi: 10.1080/07315724.1991.10718173. J Am Coll Nutr. 1991. PMID: 1955623 Review.
Cited by
-
Thrombosis and COVID-19: The Potential Role of Nutrition.Front Nutr. 2020 Sep 25;7:583080. doi: 10.3389/fnut.2020.583080. eCollection 2020. Front Nutr. 2020. PMID: 33102511 Free PMC article. Review.
-
Rectal Cancer: Redox State of Venous Blood and Tissues of Blood Vessels from Electron Paramagnetic Resonance and Its Correlation with the Five-Year Survival.Biomed Res Int. 2018 Aug 13;2018:4848652. doi: 10.1155/2018/4848652. eCollection 2018. Biomed Res Int. 2018. PMID: 30186860 Free PMC article.
-
Beneficial effects of δ-tocotrienol against oxidative stress in osteoblastic cells: studies on the mechanisms of action.Eur J Nutr. 2020 Aug;59(5):1975-1987. doi: 10.1007/s00394-019-02047-9. Epub 2019 Jul 6. Eur J Nutr. 2020. PMID: 31280345 Free PMC article.
-
Effect of palm-based tocotrienols and tocopherol mixture supplementation on platelet aggregation in subjects with metabolic syndrome: a randomised controlled trial.Sci Rep. 2017 Sep 14;7(1):11542. doi: 10.1038/s41598-017-11813-w. Sci Rep. 2017. PMID: 28912593 Free PMC article. Clinical Trial.
-
Carotenoids, vitamin A, vitamin C, vitamin E, and folate and risk of self-reported hearing loss in women.Am J Clin Nutr. 2015 Nov;102(5):1167-75. doi: 10.3945/ajcn.115.109314. Epub 2015 Sep 9. Am J Clin Nutr. 2015. PMID: 26354537 Free PMC article.
References
-
- Krauss RM, Deckelbaum RJ, Ernst N, Fisher E, Howard BV, Knopp RH, Kotchen T, Lichtenstein AH, McGill HC, Pearson TA, Prewitt TE, Stone NJ, Horn LV, Weinberg R. Dietary guidelines for healthy American adults: A statement for physicians and health professionals by the Nutrition Committee. American Heart Association. Circulation. 1996;94(7):1795–1800. - PubMed
-
- U.S Department of Agriculture. U.S. Department of Health and Human Services. Dietary Guidelines for Americans. 4. Vol. 232. Washington, DC: U.S. Printing Office, Home and Garden Bulletin No; 1995. Nutrition and Your Health.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

