Catecholamine influences on dorsolateral prefrontal cortical networks
- PMID: 21489408
- PMCID: PMC3145207
- DOI: 10.1016/j.biopsych.2011.01.027
Catecholamine influences on dorsolateral prefrontal cortical networks
Abstract
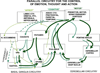
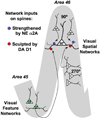
The symptoms of attention-deficit/hyperactivity disorder (ADHD) involve impairments in prefrontal cortical top-down regulation of attention and behavior. All current pharmacological treatments for ADHD facilitate catecholamine transmission, and basic research suggests that these compounds have prominent actions in the prefrontal cortex (PFC). The dorsolateral PFC is especially sensitive to levels of norepinephrine and dopamine, whereby either too little or too much markedly impairs PFC function. Recent physiological studies have shown that norepinephrine strengthens PFC network connectivity and maintains persistent firing during a working memory task through stimulation of postsynaptic α(2A)-adrenoceptors on PFC neurons. Conversely, dopamine acts at D1 receptors to narrow spatial tuning, sculpting network inputs to decrease noise (i.e., stabilization of the representation). The stimulant medications and atomoxetine appear to enhance PFC function by indirectly increasing these catecholamine actions through blockade of norepinephrine and/or dopamine transporters. In contrast, guanfacine mimics the enhancing effects of norepinephrine at postsynaptic α(2A)-receptors in the PFC, strengthening network connectivity. Stronger PFC regulation of attention, behavior, and emotion likely contributes to the therapeutic effects of these medications for the treatment of ADHD.
Copyright © 2011. Published by Elsevier Inc.
Figures


References
-
- Brozoski T, Brown RM, Rosvold HE, Goldman PS. Cognitive deficit caused by regional depletion of dopamine in prefrontal cortex of rhesus monkey. Science. 1979;205:929–931. - PubMed
-
- Arnsten AF, Berridge CW, Segal DS. Stress produces opioid-like effects on investigatory behavior. Pharmacol Biochem Behav. 1985;22:803–809. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

